What Makes a Good Leaving Group for SN1 and SN2 Reactions?
A good leaving group in both SN1 and SN2 mechanisms is one that forms a stable anion or neutral species after departure. The stability of the leaving group’s conjugate base determines its ability to leave effectively, with weaker bases generally serving as better leaving groups.
Stability of Leaving Groups
Leaving groups must stabilize the negative charge after breaking the bond with the substrate. A stable conjugate base means the leaving group can exist independently without causing high energy strain. This stability usually arises from the leaving group’s weak basicity.
- Weak bases make better leaving groups because they tolerate negative charge well.
- Good leaving groups are often the conjugate bases of strong acids.
- The ability to stabilize charge can also be enhanced by resonance or inductive effects, though this factor varies with molecular context.
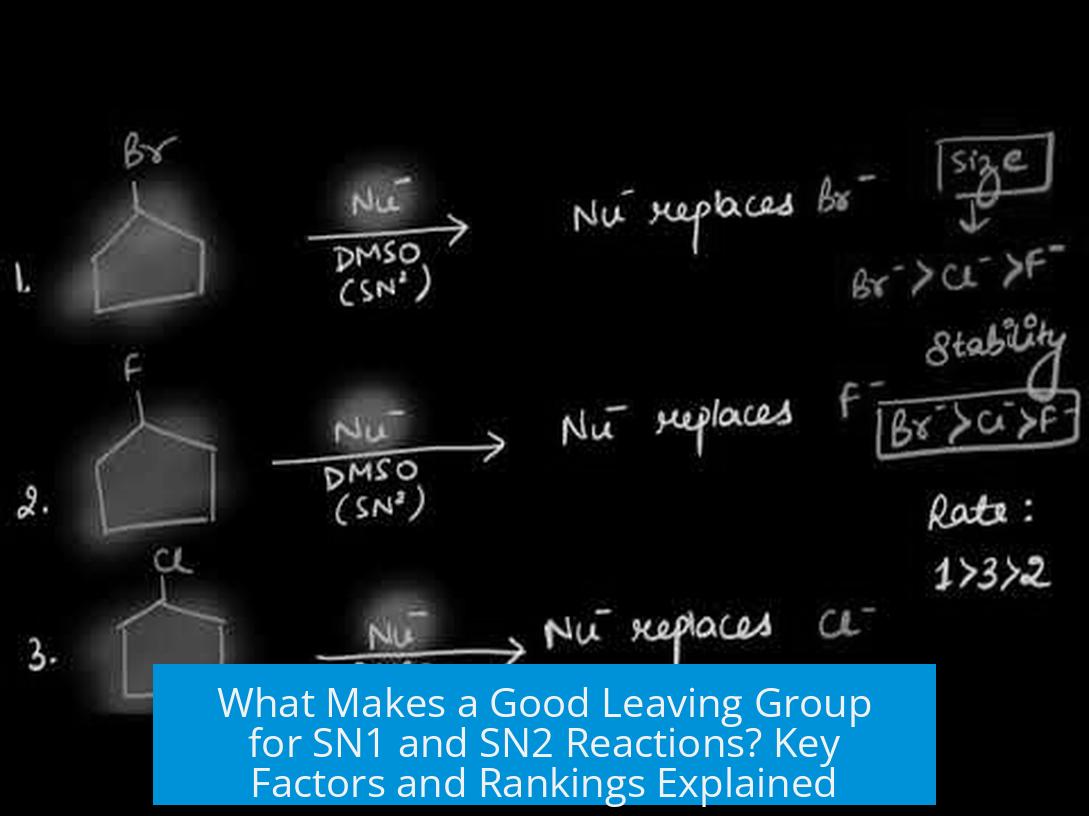
Ranking of Leaving Groups in SN2 Reactions
For SN2 reactions, the leaving groups follow a clear trend based on conjugate base stability:
| Rank | Leaving Group | Notes |
|---|---|---|
| 1 | Iodide (I−) | Best leaving group; large and polarizable |
| 2 | Bromide (Br−) | Good leaving group; less stable than iodide |
| 3 | Chloride (Cl−) | Moderate leaving group; smaller and less polarizable |
| 4 | Fluoride (F−) | Poor leaving group; high basicity and small size |
| 5 | Hydroxide (OH−), Amide (NH2−), Alkoxide (OR−) | Poor leaving groups; strong bases |
Ranking of Leaving Groups in SN1 Reactions
The leaving group trend in SN1 reactions resembles that of SN2, but strong bases like amide and alkoxide are generally not considered feasible leaving groups:
- Iodide (I−) remains the best leaving group.
- Bromide (Br−) follows closely behind iodide.
- Chloride (Cl−) also functions well but is less optimal.
- Hydroxide (OH−) is a poor leaving group due to its strong basicity.
Additional Considerations: Pi Bonds and Resonance
Although not extensively detailed, leaving groups that can stabilize through resonance or conjugation often perform better. Pi bonds adjacent to the leaving group may delocalize charge and increase leaving ability. However, specific effects depend on the molecular structure and reaction conditions.
Key Takeaways
- Good leaving groups form stable, weakly basic conjugate bases.
- For SN2: I− > Br− > Cl− > F− > OH−, NH2−, OR−.
- For SN1: I− > Br− > Cl− > OH−.
- Weak bases are better leaving groups due to better charge stabilization.
- Resonance and pi bonds may enhance leaving group stability but require context-specific evaluation.




Leave a Comment