Understanding the Reaction and the Role of KOtBu

This reaction proceeds via the formation of a sulfur ylide through the selective deprotonation of a methyl group adjacent to sulfur, enabled by KOtBu, a strong, bulky, and non-nucleophilic base; the ylide performs an intramolecular nucleophilic attack on an alkene, which leads to cyclopropane ring formation via an SN2 mechanism.
Formation of the Sulfur Ylide
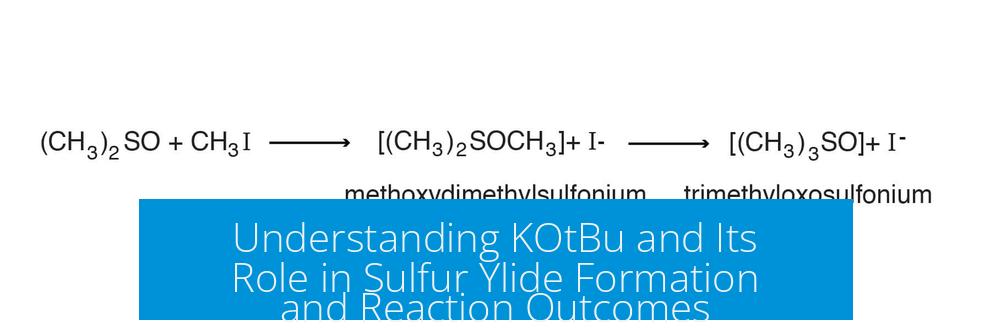
The key step initiates when KOtBu deprotonates a methyl group on the SMe2 moiety, generating a sulfur ylide. This base is ideal because its bulkiness favors selective abstraction of a methyl proton rather than more accessible protons such as those on CH2 groups.
KOtBu’s non-nucleophilic character ensures that it acts purely as a base, avoiding competing side reactions. The ylide, with its negatively charged carbon adjacent to positively charged sulfur, forms a reactive intermediate essential to the subsequent transformation.
Intramolecular Nucleophilic Attack and Cyclopropane Ring Closure
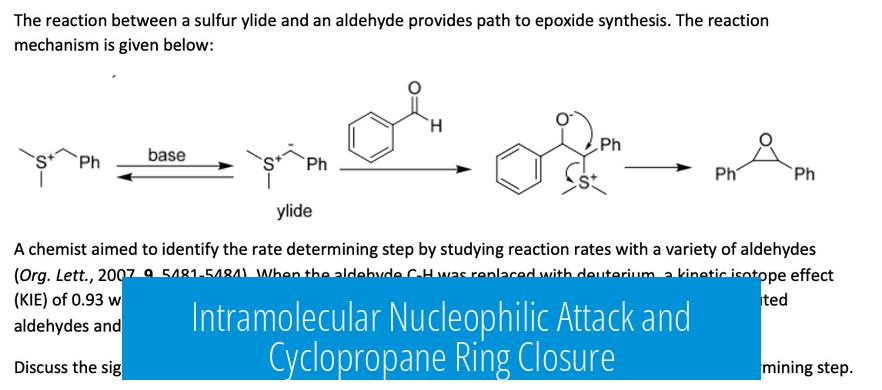
The ylide carbon functions as a nucleophile. It attacks the alkene in an intramolecular conjugate addition, triggering electron shifts that create a sulfonium cation, which is an excellent leaving group. This facilitates an SN2-like cyclization forming a three-membered cyclopropane ring.
This process uses one of the sulfur-bound methyl groups as the source of the cyclopropane unit, closing the ring efficiently and selectively.
Stereochemical Outcome and Product Profile
The reaction produces a racemic mixture with the newly formed cyclopropane ring and the thioether in syn configuration. This stereochemical result stems from the ring closure’s intramolecular nature, which controls the relative positioning of substituents but not the enantiomeric outcome.
Why KOtBu Specifically?
- Bulky base: Avoids deprotonation at less accessible sites, such as CH2 groups.
- Strong base: Efficient in abstracting the proton forming the ylide.
- Non-nucleophilic: Does not interfere with the nucleophilic steps or react with electrophilic centers.
The combination of these properties makes KOtBu optimal to initiate ylide formation selectively, which is necessary for the desired cyclopropanation.
Additional Context
This mechanism is related to the Johnson–Corey–Chaykovsky reaction, a well-studied method for cyclopropanation using sulfur ylides. The reaction exemplifies how sulfur ylides can act as versatile intermediates for ring formation, making KOtBu a common choice to facilitate such transformations.
Key Takeaways
- KOtBu acts as a strong, bulky base to selectively generate a sulfur ylide from the methyl group adjacent to sulfur.
- The sulfur ylide attacks the alkene intramolecularly, leading to cyclopropane formation via an SN2 ring closure.
- The reaction produces a racemic mixture, with syn stereochemistry between the cyclopropane and thioether groups.
- KOtBu’s non-nucleophilic nature prevents side reactions and ensures selective deprotonation.
- This process is a variant of the Johnson–Corey–Chaykovsky cyclopropanation mechanism.
What is happening in this reaction step by step?
The reaction starts by KOtBu deprotonating a methyl group near sulfur, creating a sulfur ylide. This ylide then acts as a nucleophile, attacking an alkene within the molecule. The sulfonium group leaves, and a cyclopropane ring forms through an intramolecular SN2 process.
Why is KOtBu specifically used as the base in this reaction?
KOtBu is a strong, bulky, and non-nucleophilic base. It selectively removes a proton from the methyl group next to the sulfur without touching other hydrogens, like those on CH2, ensuring ylide formation without side reactions.
What role does the sulfur ylide play in the reaction?
The sulfur ylide acts as a nucleophile after its formation. It attacks the alkene intramolecularly, initiating ring closure. This leads to a cyclopropane ring and makes the sulfonium group leave easily.
Why does this reaction produce a racemic mixture with syn stereochemistry?
The ring closing step is stereoselective but occurs equally on both enantiomeric faces. This creates a racemic mixture where the cyclopropane and thioether groups are syn, meaning they are on the same side.
How does the size and nature of KOtBu affect the reaction outcome?
Because KOtBu is bulky and non-nucleophilic, it targets only the methyl proton next to sulfur. This avoids deprotonation elsewhere, preventing competing reactions and favoring clean ylide formation and subsequent cyclopropanation.




Leave a Comment