Atomic mass is a key factor in determining the properties of an element. It is the total mass of an atom, including its protons, neutrons, and electrons. It is one of the most important components of an atom, yet it is often misunderstood. That’s why it is so important to understand what atomic mass is, how it is calculated, and how to use an atomic mass calculator to get the most accurate results.
Atomic mass can be determined by summing the number of protons and neutrons in an atom. To make this calculation easier, an atomic mass calculator can be used. This calculator takes the chemical formula of a substance and calculates the atomic mass of each element in the formula. This can help scientists and researchers determine the properties of the substance and how it could be used.
Atomic mass is an important tool for understanding the properties of an element, such as its density, melting point, and boiling point. It can also be used to determine the molecular weight of a compound, as well as the atomic weight of a molecule. With the help of an atomic mass calculator, scientists can also study how different elements interact with each other and how they could be used in various applications.
By understanding what atomic mass is and how it is calculated, scientists can get the most accurate results. This is why it is so important to understand how to use an atomic mass calculator. With this tool, scientists can study the properties of an element and the interaction between different elements in order to further their research.
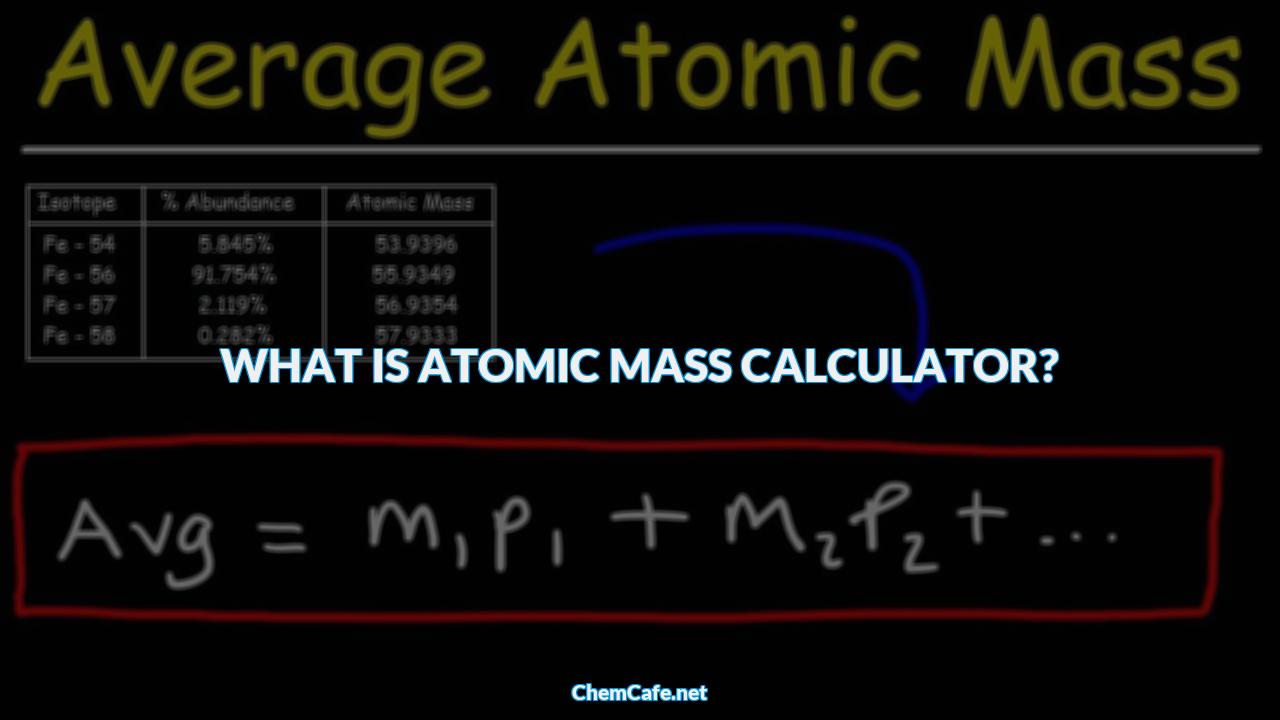
What is atomic mass calculator?
Atomic Mass Calculator is a free online tool that displays the atomic mass for the given chemical formula. Atomic Mass can be explained as the overall mass of electrons, neutrons and protons within an atom when the atom is at rest. We can calculate atomic mass by summing number of protons and neutrons in an atom.You will be able to find the atomic mass by using this below formula:
Atomic Mass = Number of Protons + Number of Neutrons
Use our below online atomic mass calculator to calculator to find atomic mass. BYJU’S online atomic mass calculator tool makes the calculation faster and it calculates the atomic mass of a chemical formula in a fraction of seconds.
How to Use the Atomic Mass Calculator?
The procedure to use the atomic mass calculator is as follows:
Step 1: Enter the chemical formula in the input field
Step 2: Now click the button “Calculate Atomic Mass” to get the result
Step 3: Finally, the atomic mass of the chemical formula will be displayed in the new window
What is Meant by Atomic Mass?
The atomic mass is defined as the mass/weight of the single atom of a chemical element. It is a unit of measurement for the mass of atoms and molecules and is expressed in unified atomic mass units (u). Atomic mass is also known as atomic weight and is represented by the symbol A. It is determined by the number of protons and neutrons present in the nucleus of the atom.
Atomic mass is an important concept in chemistry and physics. It is used in calculating the mass of a molecule, the mass of a chemical reaction, and the mass of an element. Atomic mass is also used to determine the relative abundance of elements in nature, which is an important factor in understanding the properties of molecules.
Atomic mass is also used to determine the stability of atoms and molecules. The stability of an atom or molecule is determined by the number of protons and neutrons in its nucleus. If the number of protons and neutrons is balanced, then the atom or molecule is said to be stable.
Atomic mass calculator is a useful tool for calculating the atomic mass of a chemical formula. It is a quick and easy way to determine the atomic mass of a chemical element or molecule. Atomic mass is an important concept in chemistry and physics and is used to determine the stability of atoms and molecules. It is also used to determine the relative abundance of elements in nature.
What is an atomic calculator?
An atomic calculator is a tool that helps to calculate the atomic mass of a chemical formula. It is an important tool in chemistry, as it helps to determine the mass of an atom based on its chemical elements. Atomic calculators are used in a variety of applications, such as in the analysis of organic compounds and in the study of nuclei.
Atomic calculators are often used in research, as they can provide valuable information about the composition of atoms and molecules. They can also be used to determine the mass of a molecule, the number of protons and electrons in an atom, and the atomic charge of a molecule. Atomic calculators can also be used to calculate the mass of a chemical reaction, as well as the energy produced by the reaction.
Using an atomic mass calculator is relatively simple. All you need to do is enter the chemical formula of the substance you’re analyzing. The calculator will then provide the atomic mass and other data associated with the substance.
Atomic calculators can also be used to calculate the mass of a chemical reaction. To do this, you need to enter the number of protons and electrons in the reaction. The calculator will then calculate the energy produced by the reaction and the mass of the reaction.
Atomic Charge Formula
The formula used to calculate the atomic charge of a molecule is:
AC = P – E
Where AC is the atomic charge (in eV), P is the number of protons, and E is the number of electrons. To calculate an atomic charge, simply subtract the number of electrons from the number of protons.
How to Calculate Atomic Charge?
Calculating an atomic charge is easy. All you need to do is enter the number of protons and electrons into the atomic calculator. The calculator will then calculate the atomic charge of the molecule.
Atomic calculators can also be used to calculate the mass of a chemical reaction, as well as the energy produced by the reaction. To do this, you need to enter the number of protons and electrons in the reaction. The calculator will then calculate the energy produced by the reaction and the mass of the reaction.
Atomic calculators are an important tool in chemistry and are used in a variety of applications. They can provide valuable information about the composition of atoms and molecules, as well as the energy produced by chemical reactions. They can also be used to calculate the mass of an atom or molecule, the number of protons and electrons in an atom, and the atomic charge of a molecule.
What is atomic mass and how is it calculated?
Atomic mass is an important concept in physics and chemistry, and knowing how to calculate it can be essential for understanding certain concepts in the fields. Atomic mass is the sum of the masses of the protons, neutrons, and electrons in an atom, or the average mass, in a group of atoms. This article explains what atomic mass is, how it is calculated, and why it is important.
What Is Atomic Mass?
Atomic mass is the sum of the masses of the protons, neutrons, and electrons in an atom. It is usually expressed in atomic mass units (amu). One atomic mass unit is equal to 1/12 of the mass of a single carbon-12 atom. Atomic mass is also sometimes referred to as atomic weight.
Atomic mass is an important concept in physics and chemistry because it is used to determine the relative masses of different atoms. For example, the atomic mass of hydrogen is 1 amu, while the atomic mass of oxygen is 16 amu. This means that 16 hydrogen atoms have the same mass as 1 oxygen atom.
How Is Atomic Mass Calculated?
There are two main ways to calculate atomic mass. The first is by adding the masses of the protons and neutrons in the nucleus of the atom. This is known as the “addition of protons and neutrons” method.
The second method is to calculate the average atomic mass. This is done by summing the masses of an element’s isotopes, each multiplied by its natural abundance on Earth. For example, the element carbon has two isotopes, carbon-12 and carbon-13. Carbon-12 has an atomic mass of 12 amu, and carbon-13 has an atomic mass of 13 amu. The average atomic mass of carbon is calculated by adding the contributions of both isotopes:
Average atomic mass of carbon = (12 x 0.9893) + (13 x 0.0107) = 12.011 amu
Why Is Atomic Mass Important?
Atomic mass is important because it is used to calculate the mass of atoms, molecules, and other particles. Atomic mass is also used to determine the relative masses of different elements, which is important for understanding the structure of molecules and compounds. For example, knowing the atomic masses of hydrogen and oxygen can help us understand why water has a particular formula (H2O).
Atomic mass is also important for understanding the properties of elements. For example, the atomic mass of an element affects its boiling point and melting point. This is because the heavier an element is, the more energy it takes to vaporize the element.
Atomic mass is an important concept in physics and chemistry. It is the sum of the masses of the protons, neutrons, and electrons in an atom, or the average mass, in a group of atoms. Atomic mass can be calculated by adding the masses of the protons and neutrons, or by calculating the average atomic mass of an element’s isotopes. Atomic mass is important for understanding the relative masses of different atoms, molecules, and compounds, as well as the properties of elements.
What is atomic mass and how do you calculate it?
Atomic mass is a measure of the mass of a single atom, molecule, or group of atoms. It is the sum of the masses of the protons, neutrons, and electrons in an atom, or the average mass in a group of atoms. Calculating atomic mass can help you understand the structure and properties of an atom, or a group of atoms.
Atomic mass is the sum of the masses of the protons, neutrons, and electrons in an atom, or the average mass, in a group of atoms. The atomic mass is the mass of an atomic particle or molecule. With this system, the masses of a proton and a neutron are nearly equal to 1 atomic mass unit, while the electron has a mass close to 0.0005 atomic mass unit. Atomic mass is also called atomic weight.
For atoms, the protons and neutrons of the nucleus account for almost all of the mass, and the atomic mass measured in u has nearly the same value as the mass number. Masses of fundamental atomic particles are often expressed in atomic mass units (u). “One atomic mass unit (1u) is one twelfth of the mass of an atom of carbon with six protons and six neutrons.”
How to Calculate Atomic Mass
There are several ways to calculate atomic mass. The most common method is to use the mass number (which is the total number of protons and neutrons in the nucleus) and the atomic number (the number of protons in the nucleus) of the atom.
The calculation for atomic mass is:
Atomic Mass = Mass Number – Atomic Number
For example, for an atom with a mass number of 12 and an atomic number of 6, the calculation would be:
Atomic Mass = 12 – 6
Atomic Mass = 6
Another way to calculate atomic mass is to use the average masses of the isotopes. Isotopes are atoms with the same number of protons and electrons, but different numbers of neutrons. The average mass of an element is calculated by taking the weighted average of the masses of the different isotopes.
For example, the average mass of helium is calculated by taking the weighted average of the masses of helium-3 (3.0160293 u) and helium-4 (4.0026022 u).
Average Mass of Helium = (3.0160293 x 0.000137) + (4.0026022 x 0.999863)
Average Mass of Helium = 4.002604
Atomic mass is an important concept in chemistry and physics. It is the sum of the masses of the protons, neutrons, and electrons in an atom, or the average mass in a group of atoms. There are several ways to calculate atomic mass, depending on the information you have. The most common way is to use the mass number and the atomic number of the atom. You can also calculate the average mass of an element by taking the weighted average of the masses of its isotopes.
How is atomic mass calculated?
Atomic mass is an important concept in chemistry and physics. It is the sum of the masses of the protons, neutrons, and electrons in an atom, or the average mass in a group of atoms. Knowing how to calculate atomic mass is essential for understanding the behavior of elements and compounds.
What is Atomic Mass?
Atomic mass, also referred to as atomic weight, is the sum of the masses of the protons, neutrons, and electrons in a single atom, or the average mass of a group of atoms. In chemistry, atomic mass is used to define the relative mass of an atom or element. In physics, it is used to calculate the mass of an object, such as a molecule.
Adding Protons and Neutrons
The most basic way to calculate atomic mass is by adding the masses of each proton and neutron present in the nucleus of an atom. This is done by using the number of protons and neutrons indicated in the periodic table, which is typically represented by the symbol of an element. For example, the atomic mass of carbon-12 can be calculated by adding the mass of each proton (1 amu) and neutron (1 amu) to get 12 amu, or 12 atomic mass units.
Calculating Atomic Mass
There are several ways to calculate the atomic mass of an atom. The most common method is to refer to the periodic table and find the atomic number and mass number of the element. The atomic number is the number of protons in the nucleus, and the mass number is the sum of the protons and neutrons. For example, carbon-12 has an atomic number of 6 and a mass number of 12. The atomic mass can then be calculated by subtracting the atomic number from the mass number, giving a result of 6 amu.
Mass Spectrometry
Mass spectrometry is another method used to calculate atomic mass. This technique uses a device called a mass spectrometer to measure the mass of an atom or molecule. The spectrometer uses an electric field to separate the atoms or molecules into different components, which can then be measured and used to calculate the atomic mass.
Isotope Abundance
Finally, isotope abundance can be used to calculate the atomic mass of an element. This method uses the relative abundance of different isotopes of an element to calculate the average atomic mass. Isotopes are atoms of the same element that have a different number of neutrons. For example, carbon has three common isotopes: carbon-12, carbon-13, and carbon-14. The average atomic mass of carbon is calculated by taking the relative abundance of each isotope and multiplying it by its mass.
In summary, there are several ways to calculate atomic mass. The method used will depend on the information available. For most calculations, the periodic table and mass spectrometry are the most reliable methods. Isotope abundance can also be used to calculate the average atomic mass of an element. Knowing how to calculate atomic mass is an important part of understanding the behavior of elements and compounds.
How do you find the mass of an atom?
Atomic mass is a key component of chemistry and physics, and is essential for understanding the structure of atoms and their behavior. But how do you find the mass of an atom?
There are a few different methods you can use, depending on the type of information you’re given. Here, we’ll discuss three ways to find atomic mass, and explain what atomic mass is in the first place.
Atomic mass is the total mass of all the particles in an atom, including protons, neutrons, and electrons. It’s also known as atomic weight and is measured in atomic mass units (amu).
Atomic mass is a fundamental property of an atom that is important for understanding its structure and behavior. It can be used to calculate the mass of a single atom, or a sample of atoms.
Look Up Atomic Mass on the Periodic Table
The easiest way to find the atomic mass of an element is to look it up on the periodic table. The atomic mass is listed in the bottom right corner of each element’s box.
The atomic mass is the average mass of all the atoms of the same element, taking into account the different isotopes of the element. For example, hydrogen has two isotopes, protium and deuterium, with atomic masses of 1.0078 amu and 2.0141 amu respectively. The average atomic mass of hydrogen is 1.0079 amu, taking into account the relative abundance of the two isotopes.
Calculate Atomic Mass for a Single Atom
If you know the number of protons and neutrons in an atom, you can calculate its atomic mass. This is done by adding the mass of the protons and neutrons together.
The mass of a proton is 1.0073 amu, and the mass of a neutron is 1.0087 amu. Therefore, if an atom has 6 protons and 7 neutrons, its atomic mass is 6 x 1.0073 amu + 7 x 1.0087 amu = 14.1021 amu.
Calculate Atomic Mass for a Sample
If you have a sample of atoms, you can calculate the average atomic mass of the sample. This is done by taking into account the different isotopes of the element and their relative abundances.
For example, if a sample of chlorine contains 75.77% of chlorine-35 and 24.23% of chlorine-37, the average atomic mass of the sample can be calculated by multiplying the atomic mass of each isotope by its relative abundance, and then adding the results together.
In this example, the average atomic mass of the sample is (35 x 0.7577) + (37 x 0.2423) = 35.45 amu.
Atomic mass is an important property of atoms and is essential for understanding the structure and behavior of atoms. There are several ways to calculate atomic mass, depending on the type of information you’re given. You can look up the atomic mass on the periodic table, calculate it for a single atom, or calculate it for a sample of atoms.




Leave a Comment