Valence electrons are the outermost electrons of an atom and are responsible for determining the properties of the element, such as its reactivity, hardness, and even its color. They are responsible for the number of bonds an atom can form and are integral in understanding the behavior of atoms and molecules. So, how do you find valence electrons and use them to understand the behavior of elements?
The answer lies in the periodic table. The periodic table is an arrangement of elements in order of increasing atomic number, which is the number of protons in an atom’s nucleus. This arrangement is used to categorize elements into various groups. Each group has a specific number of valence electrons, which is indicated by the group number. For example, the Group 1 elements (the alkali metals) all have one valence electron, while the Group 2 elements (the alkaline earth metals) have two valence electrons.
The total number of valence electrons of an element can also be determined by examining the vertical column in which the elements are grouped. For example, carbon (in Group 4) has four valence electrons. Similarly, nitrogen (in Group 5) has five valence electrons, and oxygen (in Group 6) has six valence electrons.
The valence electrons of an atom are important in determining its reactivity, hardness, and other properties. They are also used to determine the type of bonds the atom can form. For example, carbon is able to form four single bonds, while nitrogen is able to form three single bonds and one double bond. The valence electrons are also used to determine the valence energy level of an atom, which is the energy required to remove an electron from the outermost shell.
In summary, the number of valence electrons of an element can be determined by examining the periodic table or by looking at the vertical column in which the elements are grouped. The valence electrons are important in understanding the behavior of elements and the type of bonds they can form. Furthermore, the valence energy level of an atom can be determined by examining the number of valence electrons.
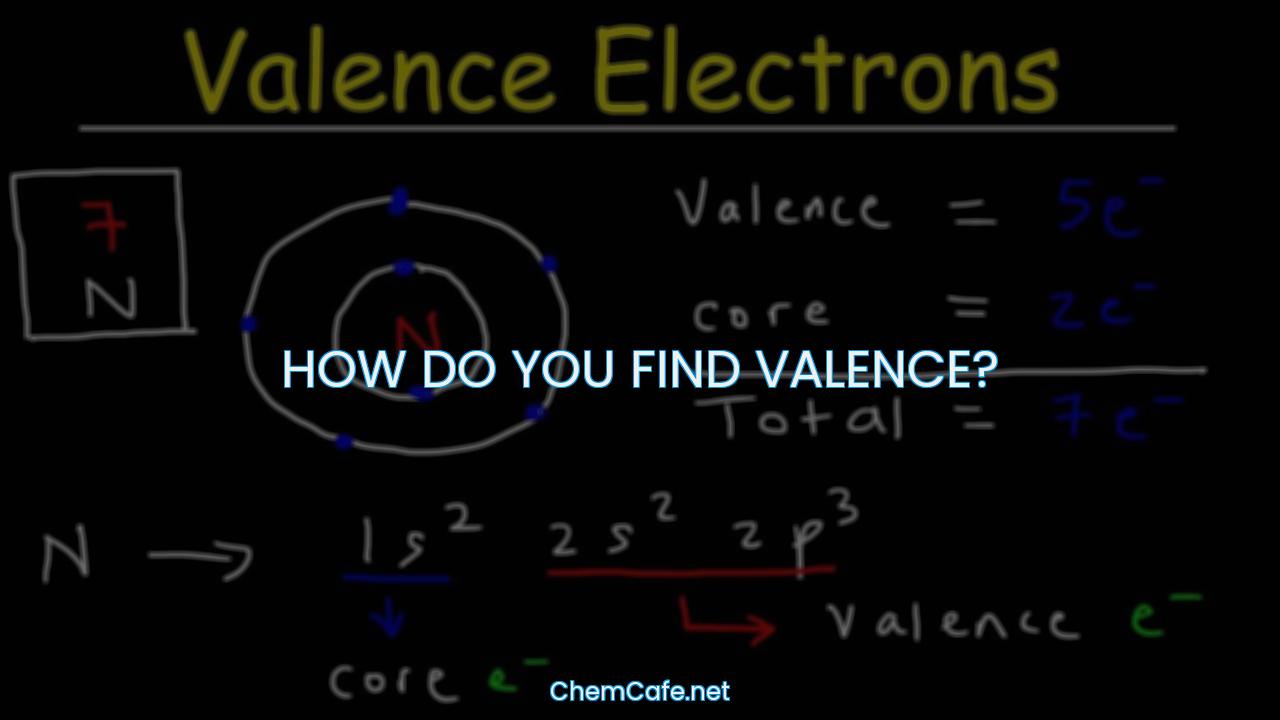
How do you find valence?
Valence is an important concept in chemistry, as it determines the type of bonds an element can form with other elements. It is located on the outermost shell (in this case, the shell resembles a ring). The term valence refers to the ability of an element to form bonds with other atoms. An element’s valence was historically determined by how many hydrogen atoms it could bond to (which is determined by how many valence electrons it has available for bonding): for example, carbon can form CH4 so it has a valence of 4, and 4 valence electrons.
Depending on the nature of elements can be metal, non-metal, or metalloid. The valence electrons of an element can be found by closely examining the vertical column in which the elements are grouped. For example, nitrogen can form NH3 so it has a valence of 3, and 3 valence electrons.
Determination of Valence Electrons
One of the easiest ways to find valence electrons is by checking out the elements’ place in the periodic table. The periodic table is a chart that arranges elements according to their atomic number, electron configuration, and chemical properties. In the periodic table, elements are arranged in vertical columns called “groups”, and each group has a specific number of valence electrons.
How many valence electrons does an element have?
You can use the periodic table to help you determine how many valence electrons an element (specifically, a neutral atom of the element) has. Look at the group that the element is in, as the group number indicates the number of valence electrons that the element has. For example, the group number of Hydrogen is 1, and it has 1 valence electron. The group number of Oxygen is 6, and it has 6 valence electrons.
You can also use the periodic table to determine the number of valence electrons of an element’s ions. Ions are atoms that have either gained or lost electrons and are therefore positively or negatively charged. Look at the group number of the element, and then add or subtract electrons from it depending on the ion’s charge.
For example, the group number of Lithium is 1, and it has 1 valence electron. If a Lithium atom has lost one electron, it becomes a positively charged ion, and it will have 0 valence electrons. Similarly, if a Lithium atom has gained one electron, it becomes a negatively charged ion, and it will have 2 valence electrons.
When determining the valence of an element, it is important to remember that the number of valence electrons is determined by the group number of the element. Knowing the group number of an element and the charge of its ion can help you determine the number of valence electrons that the element has.
Overall, valence is an important concept in chemistry as it determines the type of bonds an element can form with other elements. Valence electrons are found on the outermost shell and their number is determined by the group number of the element or its ion. Knowing the group number of an element and the charge of its ion can help you determine the number of valence electrons that the element has.
How do you find valence electrons?
The number of valence electrons in an atom is an important factor in determining its chemical properties and behavior. Valence electrons are the electrons in the outermost shell of an atom, and they are responsible for many of the atom’s properties, such as its reactivity, strength, and electrical conductivity.
Understanding valence electrons is essential for anyone trying to understand the basics of chemistry. But how do you find out how many valence electrons an atom has? Here, we explain the different ways to determine the number of valence electrons in an atom.
Determining Valence Electrons from the Periodic Table
The periodic table is a great way to find out the number of valence electrons in an atom. All elements are organized into groups, with each group having a specific number of valence electrons. For example, the group number of elements in Group 7 is 7, meaning that these elements have 7 valence electrons.
For example, the element Nitrogen (N) is in Group 5 on the periodic table. Accordingly, in order to determine its valence electrons, we must only seek the number in its ones’ place: 7. As expected, that is exactly the number of electrons in its valence shell.
This method of simply referring to the periodic table and determining the corresponding group number has eliminated the hassle and complexity that once accompanied the arduous search for individual atomic configurations.
Determining Valence Electrons of Transition Metals
What about the valence electrons of the elements in between? Depending on the nature of elements can be metal, non-metal, or metalloid. Determination of Valence Electrons One of the easiest ways to find valence electrons is by checking out the elements’ place in the periodic table. The valence electrons of an element can be found by closely examining the vertical column in which the elements are grouped. By looking at the group number that is given we can identify the number of valence electrons that an element which is listed under that specific column has.
However, if we take the transition metals (groups 3-12), finding the valence electron is quite complicated. These elements’ atomic structure is rigid and the d subshell is incomplete and sits lower than the outer shell. The number of electrons in the d subshell can be found by subtracting the group number from 10. For example, if the group number is 6, then the number of electrons in the d subshell is 4.
Determining Valence Electrons from Electronic Configuration
Another way to find or determine valence electrons is by knowing the electronic configuration. To do this, you need to know the atomic number of an element. Once you have the atomic number, you can use the periodic table to find the atomic structure of the element.
The electronic configuration of an element is its arrangement of electrons in shells. The last shell is the valence shell, and the number of electrons in it is the number of valence electrons.
Finding the valence electrons of an element is a relatively simple process. By looking at the element’s position in the periodic table or its electronic configuration, you can quickly determine the number of valence electrons it has. This knowledge is essential for understanding the chemistry of the element and its behavior in different chemical reactions.
How do you find the valence number for an element?
Atoms are composed of protons, neutrons, and electrons. The number of electrons in the outermost shell, known as the valence shell, determine the chemical properties of an element. To understand the valence number of an element, it is important to understand that the periodic table is organized in columns and rows.
The Number of Valence Electrons
The number of valence electrons of an element can be determined by the periodic table group (vertical column) in which the element is categorized. With the exception of groups 3–12 (the transition metals), the units digit of the group number identifies how many valence electrons are associated with a neutral atom of an element listed under that particular column.
For example, let’s take a look at the element Sodium (Na). Sodium is categorized under group 1 of the periodic table, shown as 1A. This tells us that Sodium has one valence electron. Similarly, Oxygen (O) is categorized under group 6 of the periodic table, shown as 6A, and it has six valence electrons.
The Valence Number
The valence number is the number of electrons that an atom can gain, lose, or share to become stable. It is determined by the number of electrons in the outermost shell of the atom.
To determine the valence number of an element, first, determine the number of valence electrons. As mentioned above, the number of valence electrons of an element can be determined by the periodic table group (vertical column) in which the element is categorized. With the exception of groups 3–12 (the transition metals), the units digit of the group number identifies how many valence electrons are associated with a neutral atom of an element listed under that particular column.
Once you have the number of valence electrons, you can use the following rule to calculate the valence number:
- If the number of valence electrons is one or two, the valence number is equal to the number of valence electrons.
- If the number of valence electrons is three, four, or five, the valence number is equal to eight minus the number of valence electrons.
- If the number of valence electrons is six, seven, or eight, the valence number is equal to the number of valence electrons minus eight.
Let’s take Oxygen as an example. Oxygen has six valence electrons. According to the rule above, the valence number of Oxygen is six minus eight, which is -2. This means that Oxygen can gain two electrons to become stable.
In summary, the valence number of an element is determined by the number of electrons in its outermost shell. To find the number of valence electrons, simply locate the column number that the element is in and use the rule mentioned above. With this information, you can now determine the valence number of any element.
How do you find the valence energy level?
Valence energy levels refer to the energy levels of electrons in an atom. These energy levels determine how an atom interacts with other atoms and how it forms chemical bonds. Understanding valence energy levels is key to understanding the behavior of atoms and molecules.
The Periodic Table
The periodic table of elements is a useful tool in determining the valence energy level. The periodic table is arranged in order of the atomic number, which is the number of protons in an atom. Each element is represented by an element symbol and its atomic number. The elements are also arranged in order of increasing atomic mass.
The valence energy level of an element can be determined by looking at its position in the periodic table. Elements at the left side of the table, such as hydrogen and helium, have the lowest valence energy levels. Elements at the right side of the table, such as chlorine and sulfur, have the highest valence energy levels.
Filling Electron Energy Levels
The valence energy level of an atom is determined by the number of electrons in its outermost energy level. This outermost level is known as the valence energy level and it is the energy level at which an atom can interact with other atoms to form chemical bonds.
The electrons in an atom fill in the energy levels according to the following pattern: The first energy level can hold up to two electrons, the second energy level can hold up to eight electrons, the third energy level can hold up to eighteen electrons, and the fourth energy level can hold up to two electrons.
Once an energy level is filled with the maximum number of electrons, the next electrons will fill the next energy level. For example, after the first energy level has two electrons (helium), the next electrons will fill the second energy level. After the second energy level has eight electrons (neon), the next electrons will fill the third energy level. This pattern continues until all of the energy levels are filled.
Calcium Valence Energy Level
Calcium is an element with the atomic number 20, which means it has 20 protons. The valence energy level of calcium is the fourth energy level, which can hold up to two electrons. This means that calcium has two electrons in its outermost energy level.
Knowing the valence energy level of an element is important in predicting its behavior and its ability to form chemical bonds. By understanding the valence energy level of an element, chemists can better predict how an atom will interact with other atoms and how it will form chemical bonds.
How do you find the valency of an element?
Valency is an important characteristic of elements and can be used to predict the behavior of a chemical compound. It is the number of electrons that an atom can gain, lose, or share with other atoms in a chemical reaction. Knowing the valency of an element is essential for understanding the reactivity of a chemical compound and for predicting its properties.
What is Valency?
Valency is the measure of an atom’s ability to form bonds with other atoms. It is determined by the number of electrons in the outermost shell of an atom. Atoms with a full outer shell of electrons are said to have a valency of zero. Atoms with one or more electrons in their outer shell have a positive valency, while atoms with one or more electrons in their outer shell have a negative valency.
The Octet Rule
The Octet Rule is a simple way to determine the valency of an element. This rule states that atoms tend to bond in such a way that their outer shell of electrons is filled with eight electrons. This is why elements in Group 8 of the periodic table (the noble gases) are inert with a valency of zero. Elements in Groups 1 and 2 have a valency of +1 and -1 respectively, as they have one and two electrons in their outer shell.
Using the Periodic Table
The position of an element in the periodic table can be used to determine its valency. Elements in Groups 1, 2, and 3 have a valency of +1, +2, and +3 respectively, as they have one, two, and three electrons in their outer shell. Elements in Groups 4, 5, 6, and 7 have a valency of -2, -3, -4, and -5 respectively.
Determining Valency with Formulae
The valency of an element can also be determined by its chemical formula. The simplest way to do this is to count the number of hydrogen atoms, chlorine atoms, or double the number of oxygen atoms that one atom of an element may combine with. For example, the formula for water is H2O, so the valency of hydrogen is +1 and the valency of oxygen is -2.
In conclusion, the valency of an element can be determined by looking at its position in the periodic table, using the Octet Rule, or by counting the number of hydrogen, chlorine, or oxygen atoms in its chemical formula. Knowing the valency of an element is essential for understanding the reactivity of a chemical compound and for predicting its properties.
How do you find the valence configuration?
Electron Configurations are essential for understanding the properties of an atom. The configuration of valence electrons in an atom determines how it interacts with other atoms, and how it behaves in chemical reactions. Knowing the valence configuration can help us to predict the properties of a group of elements and also interpret atomic spectra.
What is the Valence Configuration?
The valence configuration is the arrangement of electrons in the outermost shell of an atom. This notation for the distribution of electrons in the atomic orbitals of atoms came into practice shortly after the Bohr model of the atom was presented by Ernest Rutherford and Niels Bohr in the year 1913.
How to Find Valence Electrons?
You can easily find the valence electrons for various atoms using an orbital diagram and electron configuration. To find the valence electrons, look at the highest energy level of the atom. This is the outermost shell, and the electrons in this shell are called valence electrons.
For example, for the element nitrogen, the electron configuration is 1s2 2s2 2p3. The highest energy level is 2p3, so the valence electrons are the three electrons in this shell. In this way, each nitrogen atom will achieve an octet configuration.
Another way to find or determine valence electrons is by knowing the group number of an element in the periodic table. By looking at the group number that is given we can identify the number of valence electrons that an element which is listed under that specific column has.
Finding Valence Electrons in Compounds
In chemistry, a compound is a substance that results from a combination of two or more different chemical elements. Let’s say we have to find a number of valence electrons in a CO2 compound. The periodic table tells us that carbon has 4 valence electrons and oxygen has 6. So, for the CO2 compound, there are a total of 10 valence electrons (4 from carbon and 6 from oxygen).
Finding Valence Electrons in Transition Metals
If we take the transition metals (groups 3-12), finding the valence electron is quite complicated. These elements’ atomic structure is rigid and the d subshell is incomplete and sits lower than the outer shell. To find the number of valence electrons in transition metals, we have to subtract the total number of electrons in the s and p orbitals from the total number of electrons in the d orbital.
For example, take the element manganese (Mn). Its electron configuration is 1s2 2s2 2p6 3s2 3p6 4s2 3d5. So, the number of valence electrons in manganese is 5.
In conclusion, the valence configuration is the arrangement of electrons in the outermost shell of an atom. This notation helps us to predict the properties of a group of elements and interpret atomic spectra. You can find the valence electrons for various atoms using an orbital diagram and electron configuration, or by looking at the group number of an element in the periodic table. For transition metals, the number of valence electrons can be determined by subtracting the total number of electrons in the s and p orbitals from the total number of electrons in the d orbital.



Leave a Comment