When it comes to understanding the structure of atoms, it’s important to understand the three main particles that make up the atom: protons, neutrons, and electrons. But how do you find protons, neutrons, and electrons? Well, it’s actually quite easy.
At the most basic level, an atom is made up of a nucleus, which contains protons and neutrons, and a cloud of electrons. Protons have a positive electrical charge and a mass of 1 atomic mass unit, while neutrons have no electrical charge and a mass of 1 atomic mass unit. Electrons, on the other hand, have a negative electrical charge and a mass of about 1/1837 of an atomic mass unit.
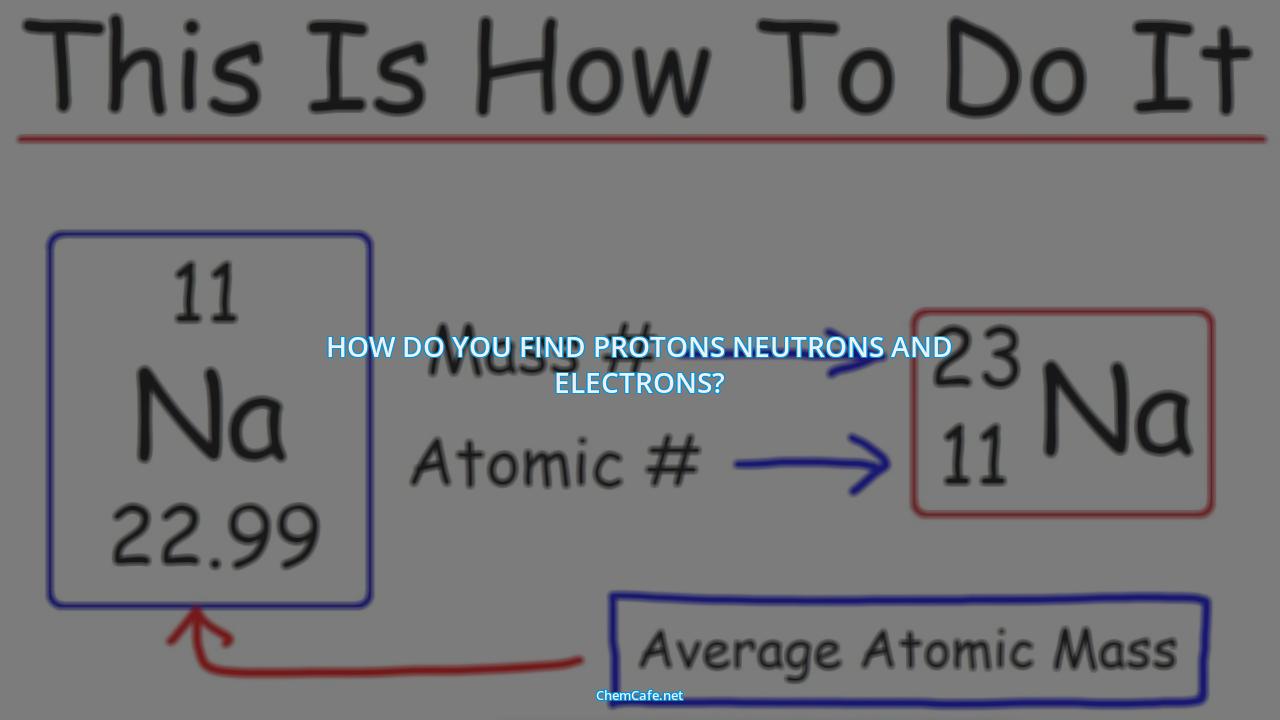
By using the Periodic Table of Elements, you can easily determine the number of protons, neutrons, and electrons in any given atom. All you have to do is look up the element on the Periodic Table and find its atomic number, which is equal to the number of protons present in the atom. The number of neutrons is equal to the mass number minus the atomic number, and the number of electrons is equal to the number of protons present.
For example, if you wanted to find out how many protons, neutrons and electrons are in an atom of krypton, you would simply look it up on the Periodic Table. The atomic number of krypton is 36, so there are 36 protons in an atom of krypton. The mass number is 84, so the number of neutrons is 84 – 36 = 48. And since the number of protons and electrons are the same, there are 36 electrons in an atom of krypton.
The same methodology can be used to find out the number of protons, neutrons and electrons in atoms of other elements, such as carbon, oxygen, neon, silver, gold, etc. So the next time you’re wondering how to find protons, neutrons, and electrons in an atom, just look up the element on the Periodic Table and follow the steps outlined above. It’s that simple!
How do you find protons neutrons and electrons?
Atoms are made up of three main particles – protons, neutrons and electrons. Protons and neutrons make up the nucleus of an atom, and the electrons orbit around it. Knowing the number of protons, neutrons and electrons in an atom can be useful when studying chemistry and physics. So, how do you find the number of protons, electrons and neutrons in an atom?
Determine Atomic Number
The first step in determining the number of protons, electrons and neutrons in an atom is to find the atomic number. This is an integer that is unique to each element, and it tells you the number of protons in the atom. The atomic number can be found on the periodic table of elements, and it is the same for all isotopes of the same element.
Calculate Neutrons and Electrons
Once you know the atomic number, you can calculate the number of neutrons and electrons. The number of neutrons is calculated by subtracting the atomic number from the mass number. The mass number is the sum of the number of protons and neutrons in the atom. The number of electrons is equal to the number of protons, because atoms are electrically neutral.
Examples
To illustrate how to calculate the number of protons, neutrons and electrons in an atom, let’s look at some examples. For example, if you have an atom of krypton, you know the atomic number is 36. This means that the atom has 36 protons. The mass number is 84, so the number of neutrons is 84 – 36 = 48. The number of electrons is also 36.
For carbon, the atomic number is 6, so there are 6 protons. The mass number is 12, so the number of neutrons is 12 – 6 = 6. The number of electrons is also 6.
For oxygen, the atomic number is 8, so there are 8 protons. The mass number is 16, so the number of neutrons is 16 – 8 = 8. The number of electrons is also 8.
Summary
Finding the number of protons, electrons and neutrons in an atom is a simple process. First, you need to determine the atomic number, which is found on the periodic table of elements. Then, you can calculate the number of neutrons and electrons by subtracting the atomic number from the mass number. Finally, the number of electrons is equal to the number of protons, since atoms are electrically neutral. With this knowledge, you can now calculate the number of protons, neutrons and electrons in any atom!
How do you find neutrons and electrons?
When it comes to understanding atoms and their components, one of the main questions is: how do you find the number of protons, neutrons, and electrons? This is a crucial step to understanding the structure of atoms and how they interact with each other.
In this article, we will go over the basics of how to determine the number of protons, neutrons, and electrons in an atom or ion. We will discuss the nuclide notation and how it can be used to calculate the number of protons, neutrons, and electrons in an atom. We will also provide a step-by-step example with a solved problem.
The Nuclide Notation
The nuclide notation is an important tool to understanding the components of atoms. It consists of three parts: the symbol of the element, the mass number, and the atomic number. The symbol of the element is the letter(s) in the middle. The mass number is the number on the left, and the atomic number is the number on the right. The mass number is the total number of protons and neutrons in an atom, while the atomic number is the number of protons.
Calculating Number of Protons and Electrons
The number of protons and electrons in an atom can be calculated by simply looking at the atomic number. The atomic number is equal to the number of protons and electrons in an atom. This means that if the atomic number is 8, the atom has 8 protons and 8 electrons.
Calculating Number of Neutrons
If we know the number of protons and the mass number of an element, we can also calculate the number of neutrons simply by subtracting its atomic number from its mass number. For example, if an element has an atomic number of 8 and a mass number of 17, the number of neutrons can be calculated as 17 – 8 = 9.
Solved Example
Let’s take a look at a solved example to better understand how to calculate the number of protons, electrons, and neutrons in an atom.
Question: An atom has an atomic number of 9 and a mass number of 19.
Answer: The atom has 9 protons, 9 electrons, and 10 neutrons.
This can be calculated by first looking at the atomic number. Since the atomic number is 9, the atom has 9 protons and 9 electrons. To calculate the number of neutrons, we subtract the atomic number from the mass number. In this case, 19 – 9 = 10. This means that the atom has 10 neutrons.
In this article, we have discussed how to calculate the number of protons, electrons, and neutrons in an atom. We discussed the nuclide notation and how it can be used to calculate the number of protons, electrons, and neutrons in an atom. We also provided an example with a solved problem. By following these steps, you will be able to find the number of protons, electrons, and neutrons in an atom or ion.
How do you figure out protons and neutrons?
Figuring out the number of protons and neutrons in an atom or ion can be a tricky task for students, but with a few simple steps, you can solve this problem in no time. In this blog post, we will discuss how to calculate the number of protons, neutrons, and electrons present in an atom or ion, along with two solved examples.
The Nuclide Notation
The nuclide notation consists of three parts – the element symbol, the atomic number, and the mass number. The element symbol is the letter(s) in the middle, which correspond to the symbol of the element. The atomic number is the number that is written before the element symbol, and this number denotes the number of protons present in the nucleus. The mass number is the number that is written after the element symbol, and this number is the sum of the number of protons and neutrons present in the nucleus.
Determine the Number of Protons, Neutrons, and Electrons
To determine the number of protons, neutrons and electrons present in an atom or ion, you need to follow the steps given below:
- Determine the number of protons present – This is easily done by simply looking at the atomic number written before the element symbol in the nuclide notation. The number of protons present is always equal to the atomic number.
- Determine the number of neutrons present – This can be done by subtracting the atomic number from the mass number. The number of neutrons present is always obtained by subtracting the atomic number from the mass number.
- Determine the number of electrons present – This number is always equal to the number of protons present. The number of electrons present is always equal to the atomic number.
Solved Example
Let’s look at an example to understand how to calculate the number of protons, neutrons, and electrons present in an atom or ion.
Question: An atom has an atomic number of 9 and a mass number of 19. How many protons, neutrons, and electrons are present?
Solution:
There are 9 protons because the atomic number is always equal to the number of protons present. There are 10 neutrons because the number of neutrons is always obtained by subtracting the atomic number from the mass number. There are also 9 electrons since the number of electrons present is always equal to the atomic number.
Figuring out the number of protons, neutrons, and electrons in an atom or ion can be a challenging task for students. However, with the help of the steps discussed in this post, you can easily calculate the number of protons, neutrons, and electrons present in an atom or ion. For further practice, you can look at the solved examples given in this post.
How do you find protons and electrons?
Atoms are the building blocks of all matter. They are made up of protons, neutrons, and electrons, and understanding how to find these components is essential for understanding how atoms form, react, and interact with one another. In this article, we will discuss how to find the number of protons, neutrons, and electrons in an atom.
What are Protons, Neutrons, and Electrons?
Protons, neutrons, and electrons are the three main particles that make up an atom. Protons have a positive electrical charge of one (left( +1 right)) and a mass of 1 atomic mass unit (left( text{amu} right)), which is about (1.67 times 10^{-27}) kilograms. They are found in the nucleus of the atom, which is a tiny, dense region at the center of the atom. Together with neutrons, they make up virtually all of the mass of an atom.
Neutrons, on the other hand, have no electrical charge and a mass of 1 amu. They are also found in the nucleus of the atom and are responsible for the stability of the nucleus. Finally, electrons have a negative electrical charge of one (left( -1 right)) and a mass of 1/1836 amu. They are found in the electron cloud which surrounds the nucleus.
How to Find the Number of Protons, Neutrons, and Electrons
The first step to finding the number of protons, neutrons, and electrons in an atom is to gather information about the element. You can do this by going to the Periodic Table of Elements and clicking on your element.
Once you have the information, the next step is to use the nuclide notation. This is a way of writing the number of protons, neutrons, and electrons in an atom. It is written as “A Z X”, where A is the mass number of the element, Z is the atomic number of the element, and X is the symbol of the element.
For example, if you are looking for the number of protons, neutrons, and electrons in an atom of carbon-12, the nuclide notation would be “12 6 C”. This tells us that there are 12 protons, 6 neutrons, and 6 electrons in an atom of carbon-12.
Examples
Let’s go through two examples together.
Example 1: Krypton
For krypton, the nuclide notation is “36 36 Kr”. This tells us that there are 36 protons, 36 neutrons, and 36 electrons in an atom of krypton.
Example 2: Oxygen
For oxygen, the nuclide notation is “16 8 O”. This tells us that there are 16 protons, 8 neutrons, and 8 electrons in an atom of oxygen.
In conclusion, protons, neutrons, and electrons are the three main particles that make up an atom. To find the number of protons, neutrons, and electrons in an atom, you can use the nuclide notation which is written as “A Z X”, where A is the mass number of the element, Z is the atomic number of the element, and X is the symbol of the element.
By following these steps and using the nuclide notation, you can easily find the number of protons, neutrons, and electrons in an atom.
How do you find protons?
Protons are a type of particle that makes up most of the mass of an atom. They are found in the nucleus of the atom, and they have a positive electrical charge of one (left( +1 right)) and a mass of 1 atomic mass unit (left( text{amu} right)). To find out the number of protons, neutrons and electrons in an atom, there are a few simple steps that you can follow.
Step 1: Gather Information
The first step is to find out some information about the element you are looking for. To do this, go to the Periodic Table of Elements and click on your element. This will give you the atomic number, which is the number of protons in the nucleus of the atom. You can also find the mass number which is the sum of the number of protons and neutrons.
Step 2: Calculate the Number of Neutrons and Electrons
Once you have the atomic number and the mass number, you can calculate the number of neutrons and electrons. To do this, subtract the atomic number from the mass number. This will give you the number of neutrons in the atom. To get the number of electrons, simply use the atomic number.
Step 3: Find the Number of Protons
The atomic number gives you the number of protons in the atom. This is because protons have a positive charge, and since atoms are neutral, the number of protons must equal the number of electrons.
Examples
Let’s take a look at two examples to help illustrate these steps.
Example 1: Oxygen
The atomic number of oxygen is 8, and the mass number is 16. To calculate the number of neutrons and electrons, we subtract the atomic number from the mass number. This gives us 8 neutrons and 8 electrons. The number of protons is equal to the atomic number, so oxygen has 8 protons.
Example 2: Krypton
The atomic number of krypton is 36, and the mass number is 84. Subtracting the atomic number from the mass number gives us 48 neutrons and 36 electrons. The number of protons is equal to the atomic number, so krypton has 36 protons.
In conclusion, it is fairly easy to find the number of protons in an atom. All you need is the atomic number and the mass number. Subtract the atomic number from the mass number to find the number of neutrons, and use the atomic number to find the number of protons and electrons.
By following these steps, you can easily find the number of protons, neutrons and electrons in any atom or ion. This is a useful skill to have when studying chemistry and physics, and it can help you understand more about the structure of atoms and how they interact with each other.
How do you find the number of electrons?
Atoms are made up of protons, neutrons, and electrons. To understand the structure of an atom and its properties, it is important to know how to find the number of protons, neutrons, and electrons. In this article, we will discuss the process of determining the number of protons, neutrons, and electrons and provide an example of how to do it.
The Nuclide Notation
The nuclide notation is a way of representing an atom or ion. It is written in the form of A Z X, where A is the mass number, Z is the atomic number, and X is the element symbol. The mass number is the sum of the number of protons and neutrons in the nucleus of an atom. The atomic number is the number of protons in the nucleus, and the element symbol is the abbreviation for the element.
Finding the Number of Protons
The number of protons in an atom is equal to the atomic number of the element. To find the atomic number, look on a periodic table. The atomic number is located in the upper left corner or is the largest number on the square. For example, let’s use oxygen as an example. According to the periodic table, oxygen has an atomic number of 8, so it has 8 protons.
Finding the Number of Neutrons
The number of neutrons can be determined by subtracting the atomic number from the mass number. The mass number is the sum of the number of protons and neutrons in the nucleus of an atom. For example, let’s use oxygen again. Oxygen has a mass number of 23 and an atomic number of 8, so the number of neutrons in oxygen is 23 – 8 = 15.
Finding the Number of Electrons
The number of electrons in an element can change, depending on if it is a neutral atom or an ion. For a neutral atom, the number of protons is exactly equal to the number of electrons. So the number of electrons is the same as the atomic number. However, it is possible to remove electrons and not change the identity of an element. These are called ions. To find the number of electrons in an ion, subtract the number of electrons removed from the number of electrons in the neutral atom. For example, let’s say that oxygen has an ion with an 11+ charge. This means that 11 electrons have been removed from the oxygen atom. The number of electrons in the neutral atom is 8, so the number of electrons in this ion is 8 – 11 = -3.
In conclusion, the process of finding the number of protons, neutrons, and electrons in an atom or ion is quite simple. First, look for the atomic number of the element in the periodic table. Then, subtract the atomic number from the mass number to find the number of neutrons. Finally, for a neutral atom, the number of electrons is equal to the atomic number. For an ion, subtract the number of electrons removed from the number of electrons in the neutral atom.


Leave a Comment