Valence electrons are the electrons that take part in the formation of a chemical bond. They are located in the outermost shell of an atom and are the outermost electrons that can participate in the formation of chemical bonds. They are an important factor in determining the chemical properties of an element. The presence of valence electrons can determine whether an element will bond with other elements.
Valence electrons are important in understanding how elements interact with each other. For example, elements in Groups 1, 2, and 13 to 17 tend to react to form a closed shell, corresponding to the electron configuration #s^2p^6#. This tendency is called the octet rule because the bonded atoms have eight valence electrons.
Knowing the number of valence electrons of an element is essential for understanding its chemical behaviour. Generally, the number of valence electrons starts at one for elements in group 1 and increases by one from left to right across each period (row) of the periodic table for groups 1–2 and 13–18. For example, aluminium has 3 valence electrons, while sodium has only 1.
The noble gases, on the other hand, have very stable electron configurations and usually do not form chemical bonds. However, some of them, such as helium, have 2 valence electrons, while others, such as argon, have 8. This makes them particularly interesting to study, as they can form compounds with other elements if they have the right number of valence electrons.
Overall, valence electrons are essential for understanding the chemical behaviour of elements. Knowing which elements have 2 valence electrons can help us better understand the interactions between elements and predict their reactions.
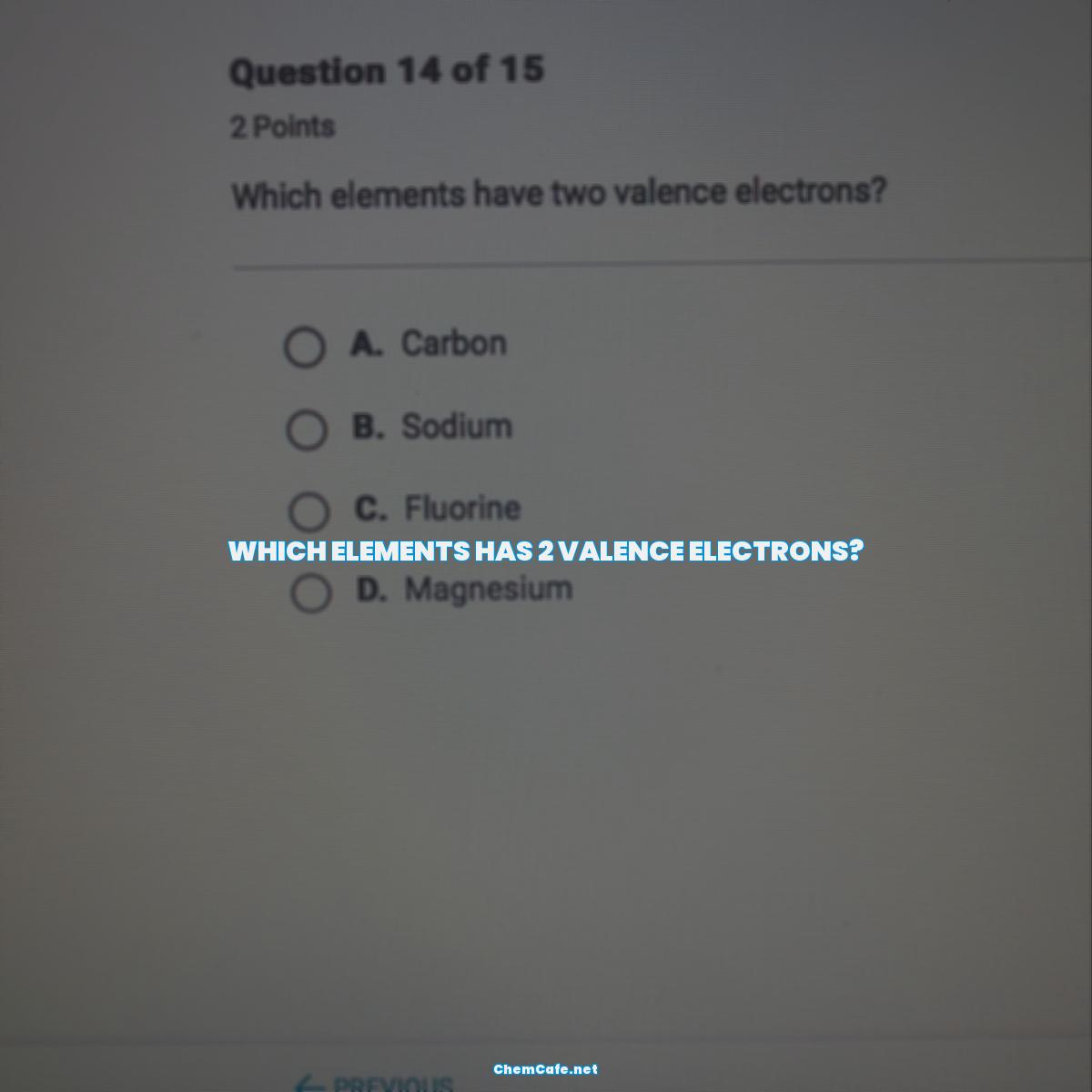
Which elements has 2 valence electrons?
Valence electrons are a crucial part of the Periodic Table. They determine the chemical properties of an element and how it will react with other elements. Valence electrons are the outermost electrons of an atom and usually the deciding factor in whether an element will form a chemical bond or not. Generally, elements in Groups 1, 2, and 13 to 17 tend to react to form a closed shell, corresponding to the electron configuration #s^2p^6#. This tendency is called the octet rule, because the bonded atoms have eight valence electrons.
How Many Valence Electrons Do Elements Have?
The number of valence electrons starts at one for elements in group 1. It then increases by one from left to right across each period (row) of the periodic table for groups 1–2 and 13–18. The maximum number of valence electrons possible is 8, which is the octet rule. This means that all elements in the same group have the same number of valence electrons.
The Elements With Two Valence Electrons
The elements that have two valence electrons are the alkaline earth metals in group 2, which are beryllium (Be), magnesium (Mg), calcium (Ca), strontium (Sr), barium (Ba), and radium (Ra). These elements are characterized by their low reactivity and high ionization energies. They are also known for their ability to form strong covalent bonds with other elements.
Aluminium and Its Valence Electrons
Aluminium has 3 valence electrons. Valence electrons are located in the outermost shell of an element. As a result, aluminium has a higher reactivity and lower ionization energy than the elements in group 2. This is because the extra electron in its outer shell can be easily removed to form a positive ion. Aluminium is also known for its ability to form strong covalent bonds with other elements.
Do Halogens Have Two Valence Electrons?
Halogens have seven valence electrons. This is because the number of valence electrons starts at one for elements in group 1 and increases by one from left to right across each period (row) of the periodic table for groups 1–2 and 13–18.
Does Sodium Have Two Valence Electrons?
Sodium has one valence electron. This is because sodium is located in group 1 and has the same number of valence electrons as the other elements in that group. As a result, sodium has a high reactivity and low ionization energy.
In conclusion, elements in group 2 have two valence electrons. These include beryllium, magnesium, calcium, strontium, barium, and radium. Aluminium has three valence electrons, while halogens have seven. Sodium has one valence electron. The number of valence electrons determines the chemical properties of an element and its ability to form strong covalent bonds with other elements.
What element has 2 electrons in the inner shell?
When it comes to understanding the structure of atoms, it’s important to know which elements have two electrons in the inner shell. This knowledge can be used to predict the chemical properties of an element and can be a useful tool for scientists.
Atoms are composed of protons and neutrons in the nucleus, and electrons in the outer shell. The number of electrons in the outer shell of an atom determines its properties. Elements with 2 electrons in the outer shell are known as Group II elements, and they have similar chemical properties.
What Elements Have Two Electrons in the Outer Shell?
The elements that have two electrons in the outer shell are magnesium (Mg), calcium (Ca), and strontium (Sr). These elements are all found in Group II, and they have similar chemical properties.
Which Electrons Have Two Electron Shells?
The electrons that have two electron shells are the electrons in the outer shell of the atom. For example, in carbon atoms, electrons are found in two electron shells. And the outermost or valence electron shell in a carbon atom is the second shell.
What Elements Have 2 Electrons in Outer Shell?
We define the “outer electron shell” as the last shell that has electrons in it, so for hydrogen, its outer electron shell would be the first shell, and for lithium its outer shell would be the second shell. Each of these elements has one electron in its outer shell, which means that they will have similar chemical properties.
Example: Oxygen
So, let’s try an example. Oxygen has an atomic number of 8, which means it has 8 protons and 8 electrons. The first shell, closest to the nucleus and with the lowest-energy electrons, is shell 1. This first shell has only one subshell (labeled 1s) and can hold a maximum of 2 electrons. This is why there are two elements in the first row of the periodic table (H & He). Because the first shell can only hold a maximum of 2 electrons, the third electron must go into the second shell.
The second shell has two subshells (labeled 2s and 2p) and can hold a maximum of 8 electrons. This is why there are eight elements in the second row of the periodic table (Li, Be, B, C, N, O, F, and Ne). So, oxygen has two electrons in its outer shell, which is the second shell.
In conclusion, elements with two electrons in their outer shell are known as Group II elements, which includes magnesium, calcium, and strontium. These elements have similar chemical properties, and they can be identified by the number of electrons in their outer shell. Oxygen, for example, has two electrons in its outer shell, which is the second shell. Understanding which elements have two electrons in the inner shell is essential for predicting the chemical properties of an element.
Which element has two valence electrons quizlet?
Valence electrons play an important role in chemical bonding. They are the outermost electrons of an atom, and they determine its chemical properties. Knowing which elements have two valence electrons is key to understanding the behavior of different elements.
The number of valence electrons for each element is determined by its position on the periodic table. Elements in the same column have the same number of valence electrons. For example, elements in the first column (sometimes labeled IA) all have one valence electron. The second column (IIA) has two valence electrons, and so on.
Boron
Boron is an element in the second column of the periodic table, so it has two valence electrons. It is the first element in this column, and it is symbolized by the letter B. Boron has 5 electrons — 2 in the first shell, and 3 in the second shell (so three valence electrons).
Argon
Argon is an element in the third column of the periodic table, so it has three valence electrons. It is the first element in this column, and it is symbolized by the letter Ar. Does argon have 2 electron shells? Argon has three electron shells. The third shell is filled with eight electrons. That is why it does not easily combine with other elements.
Helium
Helium is an element in the first column of the periodic table, so it has one valence electron. It is the first element in this column, and it is symbolized by the letter He. What element only has 2 electrons? So… for the element of HELIUM, you already know that the atomic number tells you the number of electrons.
Example: Electrons of Phosphorus
How many total and valence electrons are in a neutral phosphorus atom?
A neutral phosphorus atom has 15 total electrons. Two electrons can go into first shell, eight in the second shell, and it has five more in the third shell. The third shell is the outer valence shell, so it has 5 valence electrons.
By understanding which element has two valence electrons quizlet, you can gain a better understanding of the behavior of different elements. Knowing the number of valence electrons for each element is key for understanding the behavior of different elements. This knowledge can be useful for predicting the behavior of different elements in a variety of contexts, including chemical reactions and physical properties.
Which noble gas has 2 valence electrons?
The noble gases are located in Group 18 at the far right of the periodic table and consist of helium, neon, argon, xenon, and radon. These elements are known for their non-reactivity and full valence shells. In the case of helium, a full valence shell has two electrons, and in the remaining noble gases, eight electrons make up a full valence shell.
Do Noble Gases Have 2 Valence Electrons?
Yes, helium is the only noble gas that has two valence electrons. Since helium atoms only have two electrons and the outermost energy level is the first energy level, there can only be two valence electrons in helium. All other noble gases have eight valence electrons.
What is the Electronic Configuration of Helium?
Helium has the electronic configuration of 1s2. This means that it has two electrons in its valance shell. The first energy level is the outermost energy level and it can only hold two electrons. This is why helium is the only noble gas that has two valence electrons.
Why Are Noble Gases Non-Reactive?
Noble gases are nonreactive, nonmetallic elements in group 18 of the periodic table. This is because they have full valence shells, meaning they have eight electrons in the outermost energy level. This full valence shell makes them the least reactive of all elements.
How Can We Write Noble Gas Electronic Configurations?
An atom’s electronic configuration is the way its electrons are arranged in space based on their energies. The configuration is reflected in the element’s position in the periodic table. Because noble gases are in the last column of the periodic table and have full valence shells, the electronic configuration of other elements can be written in terms of noble gases.
For example, the electronic configuration of lithium is written as 1s2 2s1. This can be rewritten in terms of noble gases as [He] 2s1, where [He] represents the electronic configuration of helium.
In conclusion, helium is the only noble gas that has two valence electrons. All other noble gases have eight valence electrons. This is because helium atoms only have two electrons and the outermost energy level is the first energy level. The electronic configuration of other elements can be written in terms of noble gases, which have full valence shells. This full valence shell makes them the least reactive of all elements.
Does helium have 2 or 8 valence electrons?
Helium is one of the most abundant elements in the universe and is found in many different places. It’s also one of the most mysterious elements, with its unique behavior and properties. One of the most debated topics about helium is its valence electrons, and whether it has two or eight valence electrons. In this article, we’ll explore this topic in more depth and answer the question: Does helium have 2 or 8 valence electrons?
How to Find the Valence Electrons for Helium (He)
The valence electrons of an element are the electrons found in the outermost shell of the atom. For helium, the outermost shell is the first energy level, which can only hold two electrons. This means that helium only has two valence electrons.
To find out how many electrons an element has, we can look at the periodic table. Helium is in Group 8 (Nobel Gases) because its outer shell is full. The rest of the Nobel Gases have a p-orbital that can hold 6 electrons. This means that helium is the only element in Group 8 with two valence electrons.
Why does helium only have 2 valence?
The reason why helium only has two valence electrons is because the first energy level can only hold two electrons. Two completely filled orbitals means ultimate stability. Yoda would be proud.
Helium is slightly different than the other noble gas elements. It only has two electrons in its outer shell so its valence electron configuration is 1s2. Even though it only has two electrons, it is grouped with elements that have eight valence electrons. This is because the noble gases are the most stable elements with their outer shells full.
Does helium follow the octet rule? Why or why not?
The octet rule states that atoms will gain, lose, or share electrons to get 8 electrons in their outer shell. This is the most stable configuration for most elements. However, there are more exceptions to the octet rule for the noble gasses. Note that helium (He) is noble. It is element number 2 with only 2 electrons, so it cannot possibly have an octet of 8 electrons.
In conclusion, helium does not follow the octet rule and only has two valence electrons. This is because the first energy level can only hold two electrons and helium is a noble gas. Helium is the only element in Group 8 with two valence electrons, making it a very unique element.
Is the only noble gas with 2 valence electrons?
Noble gases are a group of elements found on the periodic table that have a full outer shell of electrons. They are generally considered to be unreactive and are commonly referred to as “inert gases”. Most noble gases have eight valence electrons in their outer shell, except for helium, which only has two. This begs the question, why is helium considered to be a noble gas when it has only two valence electrons, unlike the other noble gases?
Helium is the element with atomic number Z = 2. Its electronic configuration is 2. Despite having only two electrons in its outer shell, it is still considered to be a noble gas due to its inert nature. The octet rule states that atoms tend to form compounds in ways that give them eight valence electrons, and thus the electron configuration of a noble gas. However, as we can see in the case of helium, an exception to this rule can be made.
Does Helium 2+ Exist?
The helium dimer is a van der Waals molecule with formula He2 consisting of two helium atoms. It has been observed to exist in the gas phase, and was first reported in the 1960s. It is an example of a rare species in which helium has two valence electrons and can form a stable bond. The bond is relatively weak, and is formed only at very low temperatures and high pressures.
The Exception to the Octet Rule
Helium is the only noble gas with two valence electrons, but it is still considered to be a noble gas due to its inert nature. By having two electrons in its outer shell, helium is stable and does not require additional electrons to complete its octet. Elements near helium on the periodic table are also stabilized with two electrons, not an octet of eight. This is an example of an exception to the octet rule.
Helium is the only noble gas with two valence electrons, yet it is still considered to be a noble gas due to its inert nature. The octet rule states that atoms tend to form compounds in ways that give them eight valence electrons, and thus the electron configuration of a noble gas. However, an exception to this rule is made in the case of helium, which has two valence electrons. The helium dimer is a van der Waals molecule with formula He2 consisting of two helium atoms and is an example of a species in which helium can form a stable bond. Although helium has two valence electrons, it is still considered to be a noble gas due to its inert nature and its ability to form a stable bond.


Leave a Comment