Atoms are made up of three main components: protons, neutrons, and electrons. Protons have a positive charge and are located in the nucleus of the atom, while electrons have a negative charge and are found in shells, or orbitals, around the nucleus. Neutrons, on the other hand, are neutral and also located in the nucleus. Each atom can be characterized by its element’s symbol, atomic number, mass number, and charge. The symbol of the element is in the middle of the nuclide notation and the atomic number, which tells you the number of protons, is on the bottom left corner. The mass number, which is equal to the neutrons and protons added together, is on the upper left corner. Lastly, the charge is on the upper right corner.
In this post, we’ll be going over how to determine the number of protons, neutrons, and electrons in an atom or ion. We’ll first start by discussing what each of the components in the nuclide notation means. Then we’ll go through two examples together. We will also go over the rules to finding the number of protons, neutrons, and electrons. Knowing how to find the number of protons, neutrons, and electrons is important for understanding the structure of an atom and how it interacts with other atoms. It also helps when you are trying to determine the mass of an atom or the charge of an ion.
In this tutorial, you will learn how to find and calculate the number of protons, neutrons, and electrons in an atom or element. In addition, you will learn about the different subatomic particles. The atomic number of an element is simply the number of protons in its nucleus. The easiest way to find the atomic number, is to look on a periodic table, the atomic number is in the upper left corner, or is the largest number on the square. The number of protons in an atom is equal to the atomic number of the element.
The mass of a neutron is slightly greater than the mass of a proton, which is 1 atomic mass unit (left( text{amu} right)). (An atomic mass unit equals about (1.67 times 10^{-27}) kilograms.)A neutron also has about the same diameter as a proton, or (1.7 times 10^{-15}) meters. If an electron was the mass of a penny, a proton or a neutron would have the mass of a large bowling ball!
To find the number of protons, electrons and neutrons in an atom, just follow these easy steps: Step 1 – Gather Information. The first thing you will need to do is find some information about your element. Go to the Periodic Table of Elements and click on your element. If it makes things easier, you can select your element from an alphabetical listing. Use the Table of Elements to find your element’s atomic number and atomic weight. The atomic number is the number located in the upper left corner and the atomic weight is the number located on the bottom, as in this example for krypton. Step 2 – The Number of Protons is… The atomic number is the number of protons in an atom of an element.
Knowing how to find the number of protons, neutrons, and electrons is essential for understanding the structure of an atom and how it interacts with other atoms. With this knowledge, you can determine the mass of an atom or the charge of an ion. Understanding the components of an atom will allow you to further explore the complexity of the world around us.
How do you find protons neutrons and electrons?
Finding the number of protons, neutrons, and electrons in an atom or ion can be tricky, but it’s an important part of understanding chemistry. In this post, we’ll go over the basics of how to determine the number of protons, neutrons, and electrons in an atom or ion, as well as some important rules and examples.
The Nuclide Notation
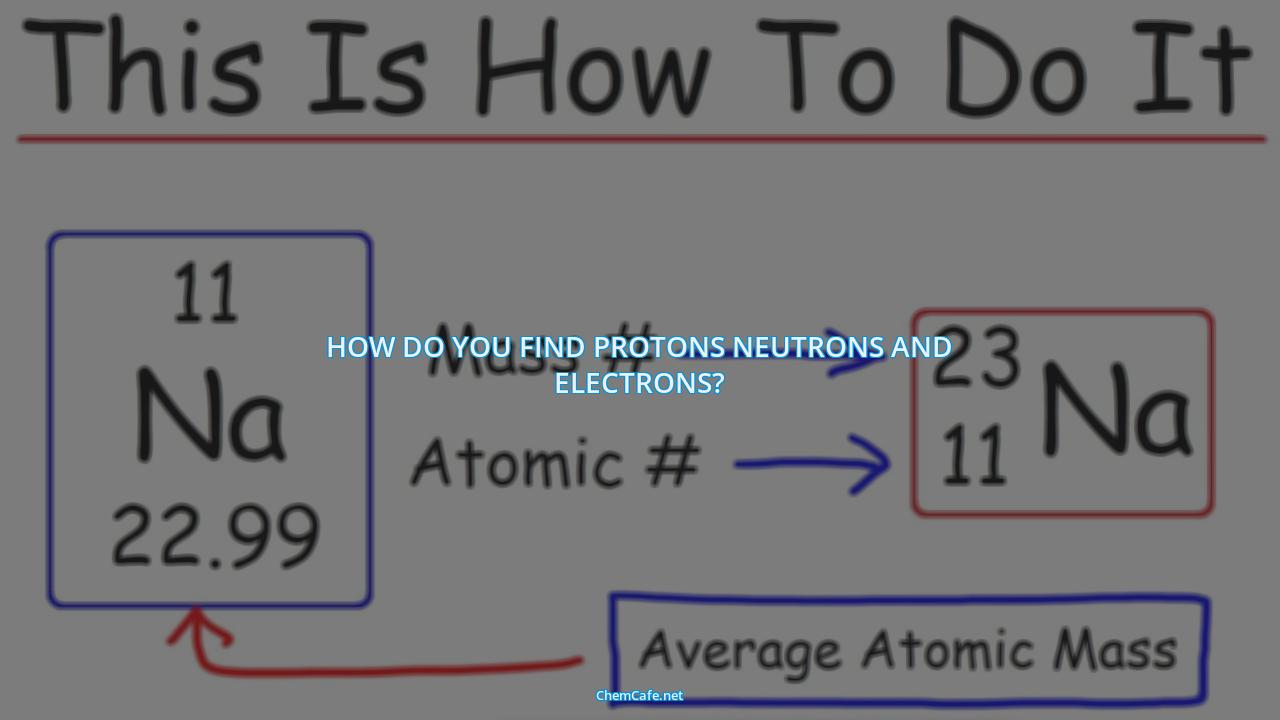
The first step to understanding how to find the number of protons, neutrons, and electrons in an atom or ion is to understand the nuclide notation. The nuclide notation consists of three parts: the symbol of the element, the atomic number, and the mass number.
The symbol of the element is located in the middle of the nuclide notation. This is the letter or group of letters that represents the element. For example, the symbol for sodium is Na.
The atomic number is located in the bottom left corner of the nuclide notation. This is the number of protons in the atom or ion. For example, the atomic number of sodium is 11.
The mass number is located in the upper left corner of the nuclide notation. This is the sum of the number of protons and neutrons in the atom or ion. For example, the mass number of sodium is 23.
Lastly, the charge is located in the upper right corner of the nuclide notation. This is the charge of the atom or ion. If there is no number or sign, then the atom or ion is neutral.
Rules to Finding Number of Protons, Neutrons, and Electrons
Once you understand the nuclide notation, finding the number of protons, neutrons, and electrons in an atom or ion becomes much easier. The rules for finding the number of protons, neutrons, and electrons in an atom or ion are as follows:
# of protons = atomic number
# of neutrons = mass number – atomic number
# of electrons = atomic number – charge
That’s it!
Examples
Let’s now apply these rules to some examples. Let’s say we want to find the number of protons, neutrons, and electrons in an atom of oxygen. The nuclide notation for oxygen is 16O. From this, we can determine that the atomic number of oxygen is 16 and the mass number is 16. Since the atom of oxygen is neutral, the charge is 0.
Using the rules above, we can determine that the number of protons in an atom of oxygen is 16, the number of neutrons is 16 – 16 = 0, and the number of electrons is 16 – 0 = 16.
Let’s try another example. Let’s say we want to find the number of protons, neutrons, and electrons in an ion of carbon. The nuclide notation for the carbon ion is 12C3+. From this, we can determine that the atomic number of carbon is 12 and the mass number is 12. Since the carbon ion has a 3+ charge, the charge is +3.
Using the rules above, we can determine that the number of protons in the carbon ion is 12, the number of neutrons is 12 – 12 = 0, and the number of electrons is 12 – +3 = 9.
Core Concepts
In this tutorial, you will learn how to find and calculate the number of protons, neutrons, and electrons in an atom or element. In addition, you will learn about the different subatomic particles. If you enjoy this tutorial, be sure to check out our others!
Vocabulary
Protons: Positively charged subatomic particles located in the nucleus of an atom.
Neutrons: Neutrally charged subatomic particles located in the nucleus of an atom.
Electrons: Negatively charged subatomic particles located in orbitals surrounding the nucleus.
Atomic Mass: A weighted average of the number of neutrons and protons present for all isotopes.
Atomic Number: Number of protons present in an atom.
Element: A pure substance that cannot be broken down into a simpler substance by chemical means.
How to find the Atomic Number
The atomic number of an element is simply the number of protons in its nucleus. The easiest way to find the atomic number is to look on a periodic table. The atomic number is in the upper left corner, or is the largest number on the square.
Finding the Number of Protons
The number of protons in an atom is equal to the atomic number of the element. If an electron was the mass of a penny, a proton or a neutron would have the mass of a large bowling ball!
Protons
A proton is one of three main particles that make up the atom. Protons are found in the nucleus of the atom. This is a tiny, dense region at the center of the atom. Protons have a positive electrical charge of one (left( +1 right)) and a mass of 1 atomic mass unit (left( text{amu} right)), which is about (1.67 times 10^{-27}) kilograms. Together with neutrons, they make up virtually all of the mass of an atom.
Neutrons
Atoms of all elements—except for most atoms of hydrogen—have neutrons in their nucleus. Unlike protons and electrons, which are electrically charged, neutrons have no charge—they are electrically neutral. That’s why the neutrons in the diagram above are labeled (n^0). The zero stands for “zero charge”. The mass of a neutron is slightly greater than the mass of a proton, which is 1 atomic mass unit (left( text{amu} right)). (An atomic mass unit equals about (1.67 times 10^{-27}) kilograms.)A neutron also has about the same diameter as a proton, or (1.7 times 10^{-15}) meters.
How many protons, electrons and neutrons are in an atom of krypton, carbon, oxygen, neon, silver, gold, etc…?
To find the number of protons, electrons and neutrons in an atom, just follow these easy steps:
Step 1 – Gather Information
The first thing you will need to do is find some information about your element. Go to the Periodic Table of Elements and click on your element. If it makes things easier, you can select your element from an alphabetical listing.
Use the Table of Elements to find your element’s atomic number and atomic weight. The atomic number is the number located in the upper left corner and the atomic weight is the number located on the bottom, as in this example for krypton:
Step 2 – The Number of Protons is…
The atomic number is the number of protons in an atom of an element. In our example, krypton’s atomic number is 36. This tells us that an atom of krypton has 36 protons in its nucleus.
The interesting thing here is that every atom of krypton contains 36 protons. If an atom doesn’t have 36 protons, it can’t be an atom of krypton.
By following the same process, you can find the number of protons, electrons, and neutrons in any atom or ion. With some practice, you’ll be able to quickly determine the number of protons, neutrons, and electrons in any atom or ion.
How do you find neutrons and electrons?
Have you ever wondered how to find the number of protons, neutrons, and electrons in an atom or ion? If so, you’re in the right place! In this post, we’ll be going over how to determine the number of protons, neutrons, and electrons. We’ll first start by discussing what each of the components in the nuclide notation means. Then we’ll go through two examples together.
The Nuclide Notation
The nuclide notation is a way of expressing the number of protons, neutrons, and electrons in an atom or ion. It is written as a letter(s) (representing the element) in the middle, with the atomic number on the bottom left corner, the mass number on the upper left corner, and the charge on the upper right corner. Let’s break it down a bit further.
Atomic Number – The atomic number is the number of protons in an atom or ion.
Mass Number – The mass number is equal to the sum of the neutrons and protons in the nucleus.
Charge – The charge tells you the amount of electrical charge on the atom or ion. If there isn’t any number or signs, then it means that atom has no charge and is neutral.
Rules to Finding Number of Protons, Neutrons, and Electrons
Now that you know the components of the nuclide notation, let’s look at the rules for finding the number of protons, neutrons, and electrons.
# of protons = atomic number
# of neutrons = mass number – atomic number
# of electrons = atomic number – charge
That’s it! Let’s apply the rules to some examples.
Examples
Let’s apply the rules to two examples.
Example 1:
Let’s look at the nuclide notation for the element Oxygen:
O-16
From this notation, we can determine that Oxygen has an atomic number of 8 and a mass number of 16. Since the charge is not given, we can assume that it is neutral, so the charge is 0.
Using the rules, we can calculate that Oxygen has 8 protons, 8 neutrons, and 8 electrons.
Example 2:
Let’s look at the nuclide notation for the element Fluorine:
F-19
From this notation, we can determine that Fluorine has an atomic number of 9 and a mass number of 19. Since the charge is not given, we can assume that it is neutral, so the charge is 0.
Using the rules, we can calculate that Fluorine has 9 protons, 10 neutrons, and 9 electrons.
Core Concepts
In this tutorial, you have learned how to find and calculate the number of protons, neutrons, and electrons in an atom or element. In addition, you have learned about the different subatomic particles.
Vocabulary
Protons: Positively charged subatomic particles located in the nucleus of an atom.
Neutrons: Neutrally charged subatomic particles located in the nucleus of an atom.
Electrons: Negatively charged subatomic particles located in orbitals surrounding the nucleus.
Atomic Mass: A weighted average of the number of neutrons and protons present for all isotopes.
Atomic Number: Number of protons present in an atom.
Element: A pure substance that cannot be broken down into a simpler substance by chemical means
How to find the Atomic Number
The atomic number of an element is simply the number of protons in its nucleus. The easiest way to find the atomic number is to look on a periodic table. The atomic number is in the upper left corner, or is the largest number on the square.
Finding the Number of Protons
The number of protons in an atom is equal to the atomic number of the element. For example, an atom of krypton has an atomic number of 36, so it has 36 protons in its nucleus.
Interesting Facts
If an electron was the mass of a penny, a proton or a neutron would have the mass of a large bowling ball!
How many protons, electrons and neutrons are in an atom of krypton, carbon, oxygen, neon, silver, gold, etc…?
To find the number of protons, electrons and neutrons in an atom, just follow these easy steps:
Step 1 – Gather Information
The first thing you will need to do is find some information about your element. Go to the Periodic Table of Elements and click on your element. If it makes things easier, you can select your element from an alphabetical listing.
Use the Table of Elements to find your element’s atomic number and atomic weight. The atomic number is the number located in the upper left corner and the atomic weight is the number located on the bottom, as in this example for krypton:
Step 2 – The Number of Protons is…
The atomic number is the number of protons in an atom of an element. In our example, krypton’s atomic number is 36. This tells us that an atom of krypton has 36 protons in its nucleus.
The interesting thing here is that every atom of krypton contains 36 protons. If an atom doesn’t have 36 protons, it can’t be an atom of krypton.
Now that you know how to find the number of protons, neutrons, and electrons, you can use the nuclide notation to find the number of each particle in any atom or ion. You can also use the rules outlined in this post to determine the number of protons, neutrons, and electrons in any atom.
How do you figure out protons and neutrons?
The protons and neutrons in an atom are two of the three fundamental particles that make up an atom. To understand the structure and behavior of atoms, it is important to know the number of protons and neutrons in the atom. In this post, we’ll discuss how to determine the number of protons, neutrons, and electrons in an atom or ion.
The Nuclide Notation
The nuclide notation is a way to represent an atom or ion. In the nuclide notation, the letter(s) in the middle is the symbol of the element. The number on the bottom left corner is the atomic number, which tells you the number of protons. The number on the upper left corner is the mass number, which is equal to the neutrons and protons added together. Lastly, the charge is on the upper right corner. If there isn’t any number or signs, then it means that atom has no charge and is neutral.
Rules to Finding Number of Protons, Neutrons, and Electrons
The rules to finding the number of protons, neutrons, and electrons are quite simple. The number of protons is equal to the atomic number, the number of neutrons is equal to the mass number minus the atomic number, and the number of electrons is equal to the atomic number minus the charge.
Examples
Let’s go through two examples together. First, let’s look at Carbon-14. Carbon-14 has an atomic number of 6, a mass number of 14, and a charge of 0. This means that Carbon-14 has 6 protons, 8 neutrons, and 6 electrons.
Next, let’s look at Bromine-35. Bromine-35 has an atomic number of 35, a mass number of 79, and a charge of -1. This means that Bromine-35 has 35 protons, 44 neutrons, and 34 electrons.
Core Concepts
In this tutorial, you will learn how to find and calculate the number of protons, neutrons, and electrons in an atom or element. In addition, you will learn about the different subatomic particles.
Covered in other articles
Vocabulary:
Protons: Positively charged subatomic particles located in the nucleus of an atom.
Neutrons: Neutrally charged subatomic particles located in the nucleus of an atom.
Electrons: Negatively charged subatomic particles located in orbitals surrounding the nucleus.
Atomic Mass: A weighted average of the number of neutrons and protons present for all isotopes.
Atomic Number: Number of protons present in an atom.
Element: A pure substance that cannot be broken down into a simpler substance by chemical means
How to find the Atomic Number
The atomic number of an element is simply the number of protons in its nucleus. The easiest way to find the atomic number, is to look on a periodic table, the atomic number is in the upper left corner, or is the largest number on the square.
Finding the Number of Protons
The number of protons in an atom is equal to the atomic number of the element. For example, an atom of krypton has 36 protons in its nucleus, because its atomic number is 36. If an atom doesn’t have 36 protons, it can’t be an atom of krypton.
To find the number of neutrons, you can simply subtract the atomic number from the mass number. If the atom is neutral, the number of electrons will be equal to the number of protons.
Remember!
- Your mass number is the total number of neutrons and protons within the atom.
- Your atomic number is the amount of protons within the atom.
- For neutral atoms, the electron number is the same as the atomic number.
Example:
This is the sodium atom. The mass number is typically found at the top (you might see it at the bottom in the periodic table) and the atomic number at the bottom.
Atomic number/number of protons = 11
Mass number/sum of protons and neutrons = 23
Number of neutrons = 23-11= 12
Number of electrons = 11 (since the sodium atom is neutral)
How many protons, electrons and neutrons are in an atom of krypton, carbon, oxygen, neon, silver, gold, etc…?
To find the number of protons, electrons and neutrons in an atom, just follow these easy steps:
Step 1 – Gather Information
The first thing you will need to do is find some information about your element. Go to the Periodic Table of Elements and click on your element. If it makes things easier, you can select your element from an alphabetical listing.
Use the Table of Elements to find your element’s atomic number and atomic weight. The atomic number is the number located in the upper left corner and the atomic weight is the number located on the bottom, as in this example for krypton:
Step 2 – The Number of Protons is…
The atomic number is the number of protons in an atom of an element. In our example, krypton’s atomic number is 36. This tells us that an atom of krypton has 36 protons in its nucleus.
The interesting thing here is that every atom of krypton contains 36 protons. If an atom doesn’t have 36 protons, it can’t be an atom of krypton. You can simply subtract the atomic number from the mass number in order to find the number of neutrons. If the atom is neutral, the number of electrons will be equal to the number of protons.
Knowing the number of protons, neutrons, and electrons in an atom is key to understanding the structure and behavior of atoms. By following the simple rules and steps discussed in this post, you can easily figure out the number of protons and neutrons in any atom or element.
How do you find protons and electrons?
Atoms are the building blocks of all matter, and understanding the components of an atom can unlock a wealth of knowledge. To understand an atom, it is important to know the number of protons, neutrons, and electrons that make it up. In this post, we’ll explain how to find the number of protons, neutrons, and electrons in an atom or ion.
The Nuclide Notation
The nuclide notation is used to identify the components of an atom. It has four parts, which are the symbol of the element in the middle, the atomic number on the bottom left corner, the mass number on the upper left corner, and the charge on the upper right corner. The atomic number tells you the number of protons, the mass number is equal to the sum of protons and neutrons, and the charge tells you the number of electrons. If there is no charge on the atom, it is considered to be neutral.
Rules to Finding Number of Protons, Neutrons, and Electrons
Using the nuclide notation, you can use three simple rules to determine the number of protons, neutrons, and electrons in an atom:
# of protons = atomic number
# of neutrons = mass number – atomic number
# of electrons = atomic number – charge
Examples
Let’s apply the rules to some examples. How many protons, electrons and neutrons are in an atom of krypton, carbon, oxygen, neon, silver, gold, etc…?
To find the number of protons, electrons and neutrons in an atom, just follow these easy steps:
Step 1 – Gather Information: The first thing you will need to do is find some information about your element. Go to the Periodic Table of Elements and click on your element. If it makes things easier, you can select your element from an alphabetical listing. Use the Table of Elements to find your element’s atomic number and atomic weight. The atomic number is the number located in the upper left corner and the atomic weight is the number located on the bottom.
Step 2 – The Number of Protons is…The atomic number is the number of protons in an atom of an element. In our example, krypton’s atomic number is 36. This tells us that an atom of krypton has 36 protons in its nucleus. The interesting thing here is that every atom of krypton contains 36 protons. If an atom doesn’t have 36 protons, it can’t be an atom of krypton.
Step 3 – The Number of Neutrons is…The number of neutrons in an atom can be calculated using the mass number and the atomic number. The mass number is the sum of protons and neutrons, and the atomic number is the number of protons. So, the number of neutrons is equal to the mass number minus the atomic number. In our example, the mass number of krypton is 84 and the atomic number is 36. So, the number of neutrons in an atom of krypton is 84 – 36 = 48.
Step 4 – The Number of Electrons is…The number of electrons in an atom can be calculated using the atomic number and the charge. The atomic number is the number of protons and the charge is the number of electrons. So, the number of electrons is equal to the atomic number minus the charge. In our example, the atomic number of krypton is 36 and the charge is 0. So, the number of electrons in an atom of krypton is 36 – 0 = 36.
Core Concepts
In this tutorial, you will learn how to find and calculate the number of protons, neutrons, and electrons in an atom or element. In addition, you will learn about the different subatomic particles.
Vocabulary
Protons: Positively charged subatomic particles located in the nucleus of an atom.
Neutrons: Neutrally charged subatomic particles located in the nucleus of an atom.
Electrons: Negatively charged subatomic particles located in orbitals surrounding the nucleus.
Atomic Mass: A weighted average of the number of neutrons and protons present for all isotopes.
Atomic Number: Number of protons present in an atom.
Element: A pure substance that cannot be broken down into a simpler substance by chemical means
How to find the Atomic Number
The atomic number of an element is simply the number of protons in its nucleus. The easiest way to find the atomic number is to look on a periodic table. The atomic number is in the upper left corner, or is the largest number on the square.
Finding the Number of Protons
The number of protons in an atom is equal to the atomic number of the element. It is not possible to know the location of an electron but only the region where it is most likely to be. The electron cloud or energy level shows the region surrounding the nucleus where the electron is most likely to be.
FAQs
Q. Why don’t the positively charged protons repel each other in the nucleus?
A. The answer is well beyond an introduction to chemistry for middle school, but one thing you can say is that there is a force called the “Strong Force,” which holds protons and neutrons together in the nucleus of the atom. This force is much stronger than the force of repulsion of one proton from another.
Q. Why doesn’t the electron smash into the proton?
A. Again, a detailed answer to this question is beyond the scope of middle school chemistry. But a simplified answer has to do with the energy or speed of the electron. As the electron gets closer to the nucleus, its energy and speed increases.
In conclusion, finding the number of protons, neutrons, and electrons in an atom or ion is a simple process once you understand the nuclide notation. All it takes is a few simple rules and you can quickly determine the components of an atom. To learn more about protons, neutrons, and electrons, be sure to check out our other articles!
How do you find protons?
Atoms are made up of protons, electrons, and neutrons. But how do you find the number of protons, electrons, and neutrons in an atom? In this article, we will discuss the steps to finding the number of protons, electrons, and neutrons in an atom.
Step 1: Gather Information
The first step in finding the number of protons, electrons, and neutrons in an atom is to gather information about the element. You can find this information on the Periodic Table of Elements. To make it easier to find the element, you can select it from an alphabetical listing. Once you have the element selected, you will want to note the atomic number and the atomic weight. The atomic number is the number in the upper left corner and the atomic weight is the number in the bottom, as in the example below for krypton:

Step 2: Find the Number of Protons
The atomic number is the number of protons in an atom of an element. In our example, krypton’s atomic number is 36. This tells us that an atom of krypton has 36 protons in its nucleus. It is important to note that every atom of krypton contains 36 protons. If an atom doesn’t have 36 protons, it can’t be an atom of krypton.
Step 3: Find the Number of Neutrons
Once you have the number of protons, you can find the number of neutrons. To find the number of neutrons, you will subtract the atomic number from the atomic weight. In our example, the atomic weight of krypton is 84. So, if we subtract 36 (the atomic number) from 84 (the atomic weight), we get 48. This tells us that an atom of krypton has 48 neutrons in its nucleus.
Step 4: Find the Number of Electrons
The last step is to find the number of electrons in an atom. To find the number of electrons, you will need to look at the charge of the element. If the element is neutral, then the number of electrons will be equal to the atomic number. In our example, krypton is neutral, so an atom of krypton has 36 electrons. If the element is not neutral, then the number of electrons will be equal to the atomic number minus the charge.
In this article, we discussed how to find the number of protons, electrons, and neutrons in an atom. We started by gathering information about the element from the Periodic Table of Elements. Then, we found the number of protons by looking at the atomic number. After that, we found the number of neutrons by subtracting the atomic number from the atomic weight. Finally, we found the number of electrons by looking at the charge of the element. With this information, you should now be able to find the number of protons, electrons, and neutrons in any atom.
How do you find the number of electrons?
If you want to know the number of electrons in an atom or ion, you must first understand the basics of atomic structure and the nuclide notation. The nuclide notation is a shorthand way of representing an atom’s structure and provides information about the number of protons, neutrons, and electrons.
The Nuclide Notation
The nuclide notation is made up of three parts. The letter(s) in the middle is the symbol of the element. The number on the bottom left corner is the atomic number, which tells you the number of protons. The number on the upper left corner is the mass number, which is equal to the neutrons and protons added together. Lastly, the charge is on the upper right corner. If there isn’t any number or signs, then it means that atom has no charge and is neutral.
Rules to Finding Number of Protons, Neutrons, and Electrons
The number of protons, neutrons, and electrons can be determined using the following rules:
# of protons = atomic number
# of neutrons = mass number – atomic number
# of electrons = atomic number – charge
Examples
Let’s look at some examples to see how this works. For a neutral atom, the number of protons is exactly equal to the number of electrons. So the number of electrons is the same as the atomic number.
However, it is possible to remove electrons and not change the identity of an element. These are called ions. The charge on the ion tells you the number of electrons. If the charge is positive, subtract that number from the atomic number to get the number of electrons. You have more protons. If the charge is negative, add the amount of charge to the atomic number to get the number of electrons. You have more electrons.
What is a Proton Number and an Ion?
Proton Number: The proton number, also called the atomic number, of an element is the number of protons in one atom of that element. The proton number can be found on the periodic table of elements.
Ion: An ion is a charged atom. In a neutral atom, the number of protons and electrons are equal. In a positively charged ion, also called a cation, the number of protons is greater than the number of electrons.
How to Determine the Number of Electrons from the Proton Number
To determine the number of electrons from the proton number, follow these steps:
Step 1: Identify the proton number, also called atomic number, of the element on the periodic table of elements.
Step 2: If it is an ion, determine the charge of the ion. This can be seen in the name of the ion.
Step 3: Determine the number of electrons:
If it is a neutral atom, the number of electrons is equal to the proton number
If the ion is positively charged, the number of electrons is found by subtracting the charge number from the proton number.
If the ion is negatively charged, the number of electrons is found by adding the charge number to the proton number.
Atoms
Here are some examples of atoms and their protons, electrons, and neutrons:
28Si: protons = 14, electrons = 14, neutrons = 14
197Au: p = 79, e = 79, n = 118
40Ar: p = 18, e = 18, n = 22
64Cu: p = 29, e = 29, n = 35
39K: p = 19, e = 19, n = 20
133Cs: p = 55, e = 55, n = 78
Ions and Atoms
Here are some examples of ions and atoms and their protons, electrons, and neutrons:
85Rb: protons = 37, electrons = 48, neutrons = 36
119Sn4+: p = 50, e = 69, n = 46
13C: p = 6, e = 7, n = 6
235U: p = 92, e = 143, n = 92
22Na+: p = 11, e = 13, n = 10
223Fr+: p = 87, e = 136, n = 86
Atomic Structure Links
To learn more about atomic structure, check out these links:
Chemical Demonstration Videos: https://study.com/academy/lesson/periodic-table-for-reference.html
How many protons, electrons and neutrons are in an atom of krypton, carbon, oxygen, neon, silver, gold, etc…?
To find the number of protons, electrons and neutrons in an atom, just follow these easy steps:
Step 1 – Gather Information: The first thing you will need to do is find some information about your element. Go to the Periodic Table of Elements and click on your element. If it makes things easier, you can select your element from an alphabetical listing. Use the Table of Elements to find your element’s atomic number and atomic weight. The atomic number is the number located in the upper left corner and the atomic weight is the number located on the bottom, as in this example for krypton:
Step 2 – The Number of Protons is… The atomic number is the number of protons in an atom of an element. In our example, krypton’s atomic number is 36. This tells us that an atom of krypton has 36 protons in its nucleus. The interesting thing here is that every atom of krypton contains 36 protons. If an atom doesn’t have 36 protons, it can’t be an atom of krypton.
As you can see, determining the number of electrons in an atom or ion is a simple process once you have a basic understanding of the nuclide notation and the rules for calculating the number of protons, neutrons, and electrons. By following the steps outlined in this post, you should be able to easily find the number of electrons in any atom or ion.
How do you find protons and neutrons?
Atoms are made up of three primary particles: protons, neutrons, and electrons. Knowing the number of protons, neutrons, and electrons in an atom is essential for understanding its properties. But how do you find the number of protons and neutrons in an atom? In this post, we’ll cover the basics of how to find the number of protons, neutrons, and electrons in an atom or ion.
The Nuclide Notation
The nuclide notation is a shorthand way of representing an atom’s composition. It contains four pieces of information: the element’s symbol, the atomic number, the mass number, and the charge. The letter(s) in the middle is the symbol of the element. The number on the bottom left corner is the atomic number, which tells you the number of protons. The number on the upper left corner is the mass number, which is equal to the neutrons and protons added together. Lastly, the charge is on the upper right corner. If there isn’t any number or signs, then it means that atom has no charge and is neutral.
Rules to Finding Number of Protons, Neutrons, and Electrons
Figuring out the number of protons, neutrons, and electrons in an atom is a matter of understanding the nuclide notation and applying some simple rules. The rules are:
- Number of protons = atomic number
- Number of neutrons = mass number – atomic number
- Number of electrons = atomic number – charge
Examples
Now that we know the rules, let’s apply them to some examples. For instance, how many protons, electrons and neutrons are in an atom of krypton, carbon, oxygen, neon, silver, gold, etc.? To find the number of protons, electrons and neutrons in an atom, just follow these easy steps:
Step 1 – Gather Information
The first thing you will need to do is find some information about your element. Go to the Periodic Table of Elements and click on your element. If it makes things easier, you can select your element from an alphabetical listing. Use the Table of Elements to find your element’s atomic number and atomic weight. The atomic number is the number located in the upper left corner and the atomic weight is the number located on the bottom, as in this example for krypton:
Step 2 – The Number of Protons is…
The atomic number is the number of protons in an atom of an element. In our example, krypton’s atomic number is 36. This tells us that an atom of krypton has 36 protons in its nucleus. The interesting thing here is that every atom of krypton contains 36 protons. If an atom doesn’t have 36 protons, it can’t be an atom of krypton.
Step 3 – The Number of Neutrons is…
We already know the atomic number (36) and the mass number (84). So, to find the number of neutrons, we just need to subtract the atomic number from the mass number. In our example, the difference is 48. This tells us that an atom of krypton has 48 neutrons in its nucleus.
Step 4 – The Number of Electrons is…
To find the number of electrons, we need to subtract the charge from the atomic number. Since there is no charge in our example, we don’t need to make any subtraction. This tells us that an atom of krypton has 36 electrons in orbit around its nucleus.
Core Concepts
In this tutorial, you will learn how to find and calculate the number of protons, neutrons, and electrons in an atom or element. In addition, you will learn about the different subatomic particles. If you enjoy this tutorial, be sure to check out our others!
Vocabulary
- Protons: Positively charged subatomic particles located in the nucleus of an atom.
- Neutrons: Neutrally charged subatomic particles located in the nucleus of an atom.
- Electrons: Negatively charged subatomic particles located in orbitals surrounding the nucleus.
- Atomic Mass: A weighted average of the number of neutrons and protons present for all isotopes.
- Atomic Number: Number of protons present in an atom.
- Element: A pure substance that cannot be broken down into a simpler substance by chemical means.
How to find the Atomic Number
The atomic number of an element is simply the number of protons in its nucleus. The easiest way to find the atomic number, is to look on a periodic table, the atomic number is in the upper left corner, or is the largest number on the square.
Finding the Number of Protons
The number of protons in an atom is equal to the atomic number of the element. If an electron was the mass of a penny, a proton or a neutron would have the mass of a large bowling ball!
Protons
A proton is one of three main particles that make up the atom. Protons are found in the nucleus of the atom. This is a tiny, dense region at the center of the atom. Protons have a positive electrical charge of one (left( +1 right)) and a mass of 1 atomic mass unit (left( text{amu} right)), which is about (1.67 times 10^{-27}) kilograms. Together with neutrons, they make up virtually all of the mass of an atom.
Neutrons
Atoms of all elements—except for most atoms of hydrogen—have neutrons in their nucleus. Unlike protons and electrons, which are electrically charged, neutrons have no charge—they are electrically neutral. That’s why the neutrons in the diagram above are labeled (n^0). The zero stands for “zero charge”. The mass of a neutron is slightly greater than the mass of a proton, which is 1 atomic mass unit (left( text{amu} right)). (An atomic mass unit equals about (1.67 times 10^{-27}) kilograms.)A neutron also has about the same diameter as a proton, or (1.7 times 10^{-15}) meters.
Knowing the number of protons and neutrons in an atom is an essential part of understanding its properties. With the nuclide notation and the rules outlined in this post, you should now have a clear understanding of how to find the number of protons and neutrons in an atom.




Leave a Comment