Have you ever wondered why atoms always contain the same number of electrons and protons? It turns out that protons and electrons are not always equal in number, although the state of having the same number of each is common. In this article, we will explore the science behind why protons and electrons are not always equal, and why they often are.
Atoms are composed of three types of particles: protons, electrons and neutrons. Protons carry a positive charge, electrons carry a negative charge, and neutrons have no charge. Protons and neutrons have approximately the same mass, but electrons are much lighter. This difference in mass between protons and electrons is key in understanding why protons and electrons are not always equal in number.
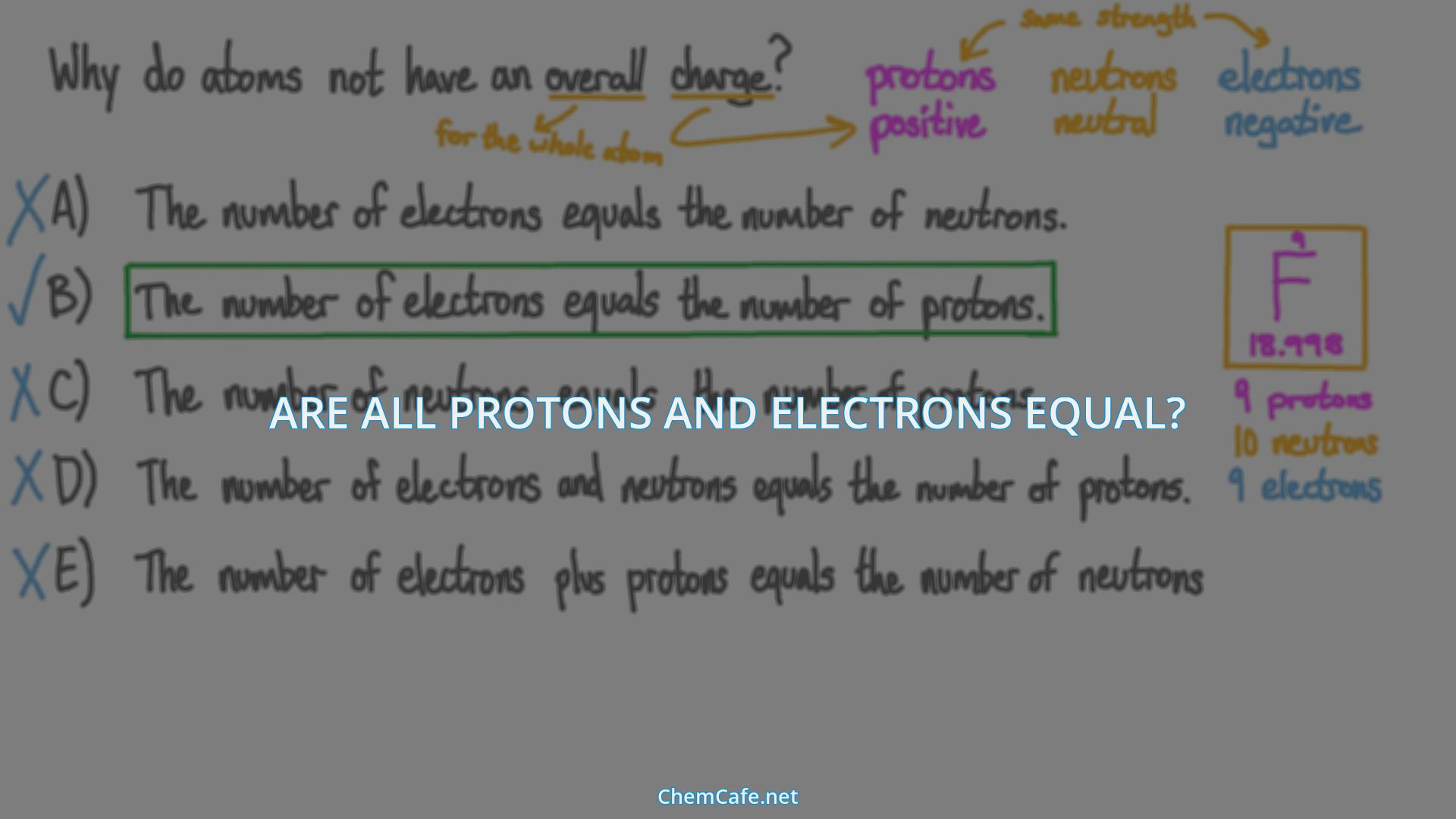
The positive charge on a proton is equal in magnitude to the negative charge on an electron. This means that in order for an atom to be neutral, it must have an equal number of protons and electrons. If an atom has an unequal number of protons and electrons, then the atom will have a net charge. This is why atoms often contain the same number of electrons and protons, and is referred to as the Atom’s Neutrality Principle.
However, atoms without an equal number of electrons and protons are more common than many people realize. For example, the atoms found in table salt have an unequal number of protons and electrons. It’s also important to note that the atomic mass unit (amu) is a unit of mass equal to one-twelfth the mass of a carbon-12 atom.
So, while protons and electrons are not always equal in number, it is often the case that atoms contain the same number of each. Understanding the science behind this phenomenon is key in understanding the behavior of atoms.
Are all protons and electrons equal?
The answer to this question is not as simple as it may seem. While it is true that protons and electrons carry equal amounts of charge, they are not the same in terms of mass and other properties. To understand why, it is important to first look at the three main components of an atom: protons, neutrons, and electrons.
Protons and Neutrons
Protons are positively charged particles that are found in the nucleus of an atom. They have an atomic number of one, which is the same as the number of protons in an atom. Neutrons, on the other hand, are neutral particles that are also found in the nucleus. They do not carry any charge and have an atomic number of zero. Protons and neutrons are much heavier than electrons, with a mass of approximately 2000 times that of an electron.
Electrons
Electrons are negatively charged particles that are found in the orbitals of an atom. They have an atomic number of minus one, which is the same as the number of electrons in an atom. Electrons are much lighter than protons and neutrons, with a mass of approximately one-thousandth that of a proton.
Atomic Mass Unit (amu)
The atomic mass unit (amu) is a unit of mass equal to one-twelfth the mass of a carbon-12 atom. This is the standard unit used to measure the mass of atoms and molecules. It is also used to calculate the number of protons and electrons in an atom.
Atoms with Unequal Proton and Electron Counts
Atoms without an equal number of protons and electrons are more common than many people realize, such as the atoms found in table salt. Salt consists of the elements sodium and chlorine, both of which have an unequal number of protons and electrons. Sodium has 11 protons and 12 electrons, while chlorine has 17 protons and 18 electrons. This means that the atoms in salt are not neutral, and they have a net charge of either positive or negative.
In summary, protons and electrons do not always have equal numbers in an atom. While it is true that they carry equal amounts of charge, they are not the same in terms of mass and other properties. Atoms with unequal numbers of protons and electrons are more common than many people realize, such as the atoms found in table salt. Understanding the three components of an atom, and how they interact, is essential for anyone interested in chemistry.
Are protons and electrons equal?
At the core of all matter, protons and electrons are the two primary particles that make up the atoms that make up all matter. But while they may seem similar at first glance, these two particles are actually quite different.
The Mass Difference
The most obvious difference between protons and electrons is their mass. Protons and neutrons have approximately the same mass, but they are both much more massive than electrons (approximately 2,000 times as massive as an electron).
The Charge Difference
The other major difference between these two particles is their charge. The positive charge on a proton is equal in magnitude to the negative charge on an electron. As a result, a neutral atom must have an equal number of protons and electrons.
The Atomic Mass Unit
The atomic mass unit (amu) is a unit of mass equal to one-twelfth the mass of a carbon-12 atom. This means that the mass of one proton is equal to 1.007 amu, while the mass of one electron is equal to just 0.0005 amu.
The Attraction of Opposites
Since opposite charges attract each other, the negatively charged electrons are attracted to the positively charged protons. Tell students that this attraction is what holds the atom together. In other words, a neutral atom must have exactly one electron for every proton. If a neutral atom has 1 proton, it must have 1 electron. If a neutral atom has 2 protons, it must have 2 electrons. If a neutral atom has 10 protons, it must have 10 electrons. You get the idea. In order to be neutral, an atom must have the same number of electrons and protons.
In conclusion, protons and electrons are not equal. They have different masses and different charges. But they are both essential to the structure of atoms, and they work together to form a neutral atom. The attraction of opposite charges is what holds atoms together and gives them their stable structure.
Protons and electrons may not be equal, but they are both essential to the structure of atoms. Understanding the differences between these two particles can help us better understand the building blocks of matter.
Why are protons and electrons not equal?
Atoms are composed of protons, neutrons, and electrons. Protons and neutrons have approximately the same mass, but they are both much more massive than electrons (approximately 2,000 times as massive as an electron). The positive charge on a proton is equal in magnitude to the negative charge on an electron. As a result, a neutral atom must have an equal number of protons and electrons.
Atoms without an equal number of electrons and protons are more common than many people realize, such as the atoms found in table salt. When an atom or group of atoms has either gained or lost some electrons, this is known as an ION. Ions can occur when atoms interact with one another, and the number of electrons does not necessarily equal the number of protons.
What is an ION?
An ION is an atom (or group of atoms) that has either gained or lost electrons, resulting in an unequal number of protons and electrons. This creates a net positive or negative charge, depending on whether electrons were gained or lost. This can happen when atoms interact with one another, and the resulting ION is more stable than the original atom.
Why do Ions form?
Ions form because atoms gain or lose electrons in order to reach a more stable state. This is due to the fact that the electron configuration of an atom is determined by the number of protons in its nucleus and the number of electrons surrounding it. If an atom has too few or too many electrons, it can become unstable and seek to reach a more balanced state by either gaining or losing electrons.
What is an Atomic Mass Unit (amu)?
The atomic mass unit (amu) is a unit of mass equal to one-twelfth the mass of a carbon-12 atom. This is used to measure the relative masses of different atoms. The mass of a proton or neutron is slightly larger than one amu, whereas the mass of an electron is much smaller than one amu.
Atoms do not always contain the same number of electrons and protons, although this state is common. When atoms interact with one another, they can gain or lose electrons and form an ION. This is because atoms seek to reach a more stable state by either gaining or losing electrons, depending on the number of protons in its nucleus. The atomic mass unit (amu) is a unit of mass used to measure the relative masses of different atoms, and it is slightly larger than the mass of a proton or neutron, but much smaller than the mass of an electron.
What is it called when protons and electrons are equal?
Atoms are the building blocks of all matter, and they are composed of protons, neutrons and electrons. Protons and electrons have an interesting relationship: they have opposite electrical charges, meaning that the positively charged protons are attracted to the negatively charged electrons. But, in order for an atom to be neutral, the number of protons and electrons must be equal. This phenomenon is known as charge neutrality.
Charge Neutrality
Charge neutrality is a term used to describe an atom that has the same amount of protons and electrons. In a neutral atom, the positive charges of the protons are exactly equal to the negative charges of the electrons. As a result, the atom has no overall charge.
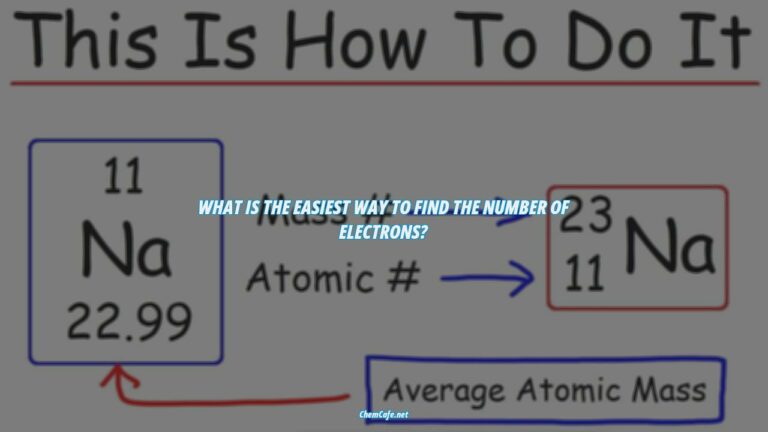
The number of protons and electrons in an atom can be determined by looking at the atomic number. The atomic number is the number of protons in the nucleus of an atom, and it is also the number of electrons in a neutral atom. For example, the atomic number of carbon is 6, so a neutral carbon atom will have 6 protons and 6 electrons.
The Role of Neutrons
Neutrons play an important role in charge neutrality, as they help to balance out the number of protons and electrons. Neutrons do not have an electrical charge, so they do not affect the overall charge of an atom. However, they do have mass, and they can help to balance out the number of protons and electrons.
For example, the atomic number of oxygen is 8, so a neutral oxygen atom will have 8 protons and 8 electrons. However, oxygen atoms usually have 8 neutrons as well, which helps to balance out the number of protons and electrons. Without the neutrons, the oxygen atom would be positively charged, because there would be more protons than electrons.
Importance of Charge Neutrality
Charge neutrality is important for atoms to function properly. If an atom has too many protons or electrons, it becomes charged, which can affect the way it interacts with other atoms. For example, a positively charged atom will be attracted to a negatively charged atom, and vice versa.
Charge neutrality is also important for chemical reactions. When two atoms come into contact with each other, they will form a chemical bond if their electrical charges are equal. If the charges are not equal, the atoms will repel each other.
Charge neutrality is an important concept in chemistry, and it is the reason why atoms have the same number of protons and electrons. Neutrons also play an important role in charge neutrality, as they help to balance out the number of protons and electrons. Without charge neutrality, atoms would not be able to form chemical bonds and chemical reactions would not be possible.
Are protons and electrons equal in charge?
Atoms are the building blocks of all matter, and they are composed of three main types of particles: protons, neutrons, and electrons. The mass of an electron is only about 1/2000 the mass of a proton or neutron, so electrons contribute virtually nothing to the total mass of an atom. Electrons have an electric charge of (-1), which is equal but opposite to the charge of a proton, which is (+1). All atoms have the same number of electrons as protons, so the positive and negative charges “cancel out”, making atoms electrically neutral.
To visualize this, we can project the animation Protons and Electrons. This animation shows two protons repelling each other and two electrons repelling each other. However, a proton and an electron attract each other. In other words, the same or “like” charges repel one another and opposite charges attract one another.
Since opposite charges attract each other, the negatively charged electrons are attracted to the positively charged protons. This attraction is what holds the atom together. Electrons are one of three main types of particles that make up atoms. Unlike protons and neutrons, which consist of smaller, simpler particles, electrons are fundamental particles that do not consist of smaller particles. They are a type of fundamental particle called leptons. All leptons have an electric charge of (-1) or (0). Electrons are extremely small.
What is the charge of an electron?
The charge of an electron is always (-1). This is the same as the charge of a proton, which is (+1). All atoms contain the same number of electrons and protons, so the positive and negative charges cancel out, making atoms electrically neutral.
How does the charge of protons and electrons affect atoms?
The charge of protons and electrons has a great effect on atoms. As mentioned above, the attraction between the negatively charged electrons and the positively charged protons is what holds the atom together. The charge of the electrons also determines the chemical properties of the atom, as electrons are responsible for forming chemical bonds with other atoms.
Are there any other particles with an electric charge?
Yes, there are other particles with an electric charge. Neutrons have no charge, but there are also particles called positrons and antiprotons that have a charge of (+1) and (-1), respectively. These particles are created in high-energy particle accelerators and are used in research.
In conclusion, the charge of protons and electrons is equal in magnitude, but opposite in sign. This is what makes atoms electrically neutral and allows them to form chemical bonds. There are also other particles with electric charge, such as positrons and antiprotons. Understanding the charge of protons and electrons is essential to understanding the structure and properties of atoms.
Are electrons and protons the same?
Atoms are comprised of protons, electrons and neutrons. Protons have a positive charge (+) and electrons have a negative charge (-). The positive charge of the protons is equal to the negative charge of the electrons. Opposite charges attract each other, and this attraction is responsible for holding the atom together.
The Same and Opposite Charges in Atoms
Let’s explore the idea of same and opposite charges. When two protons come together they repel each other because they both have a positive charge. Similarly, when two electrons join together they repel each other because they both have a negative charge. In contrast, when a proton and an electron come together, the opposite charges attract each other.
Why Do Atoms Always Contain the Same Number of Electrons and Protons?
Atoms without an equal number of electrons and protons are more common than many people realize, such as the atoms found in table salt. This is why atoms do not always contain the same number of electrons and protons, although this state is common.
The number of protons in an atom determines its kind, or element. For example, hydrogen has one proton, helium has two protons, and so on. The number of protons in an atom is fixed and is the same as the number of electrons. This is why atoms contain the same number of electrons and protons, because they need to be in balance in order to form a stable atom.
The neutrons, which carry no charge, can vary in number. The number of neutrons affects the atom’s mass, but not its identity. In other words, an atom with one proton, one electron, and one neutron is still hydrogen, but it is a different isotope of hydrogen.
Protons and Electrons in Action
Project the animation Protons and Electrons and explain to students that two protons repel each other and that two electrons repel each other. But a proton and an electron attract each other. Another way of saying this is that the same or “like” charges repel one another and opposite charges attract one another.
Since opposite charges attract each other, the negatively charged electrons are attracted to the positively charged protons. Tell students that this attraction is what holds the atom together.
To summarize, atoms contain the same number of electrons and protons because they need to be in balance in order to form a stable atom. Opposite charges attract each other, while same charges repel each other. This is why protons and electrons attract each other and form the basis of an atom.
Atoms do not always contain the same number of electrons and protons, although this state is common. The number of protons in an atom determines its kind, or element. The number of neutrons affects the atom’s mass, but not its identity. Protons and electrons interact through the same and opposite charges, and this attraction is what holds the atom together.


Leave a Comment