Want to know the easiest way to find the number of electrons in an atom? You’ve come to the right place! From determining the atomic number to counting electrons in a molecule, this article will help you find the number of electrons in an element quickly and easily.
Atoms are made up of protons, neutrons, and electrons. The atomic number of an element (Z) is equal to the number of protons in the nucleus. This makes it easy to find the number of electrons in a neutral atom. All you need to do is match the number of protons with the number of electrons. Additionally, the mass number (M) of an atom is equal to the total number of protons and neutrons in the nucleus. This means the number of neutrons is equal to the difference between the mass number and atomic number.
To find the number of valence electrons (VE), you need to look at the outer shell of the atom. This is the number of electrons that are involved in chemical reactions. By knowing the number of valence electrons, you can determine the reactivity of the atom.
If you are struggling to find the number of electrons without a periodic table, there is an easy way to do it. Just compare the size of an electron to the nucleus of an atom with the earth to the sun. This will give you a good estimate of the number of electrons in the atom.
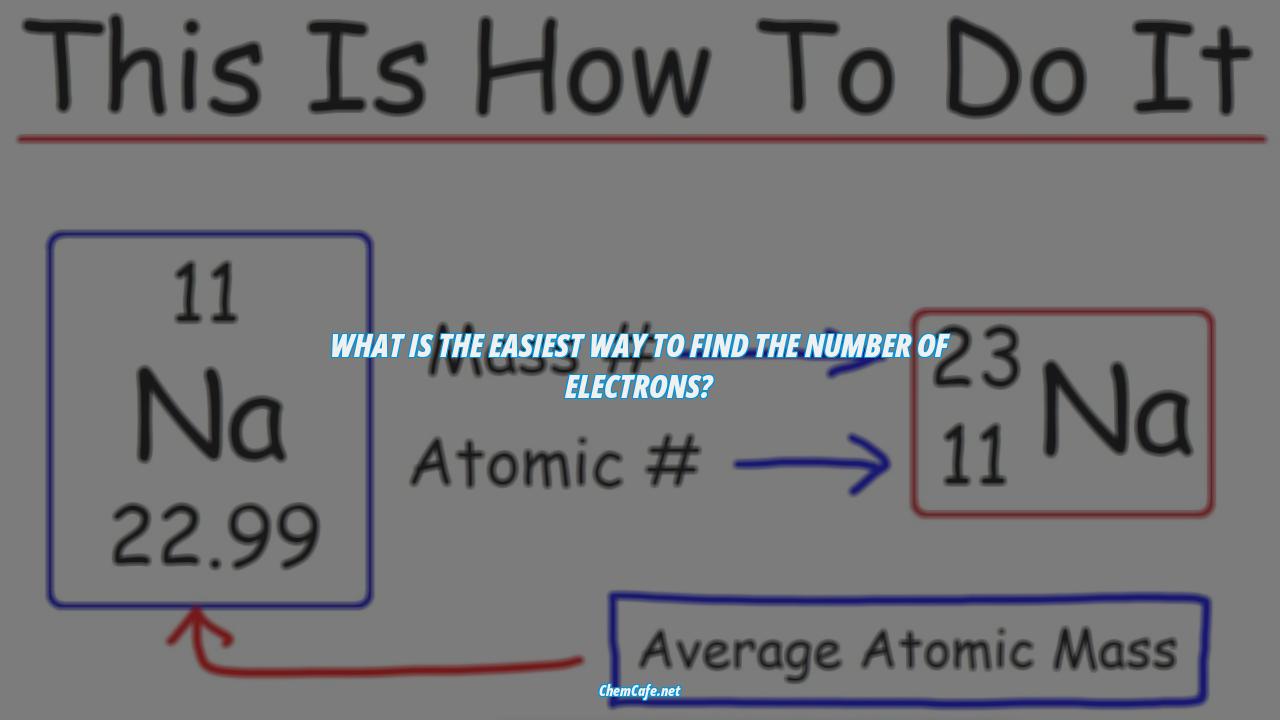
Using the periodic table is by far the easiest way to find the number of electrons in an element. All you have to do is locate the element’s atomic number in the upper left-hand corner of the square. This will automatically tell you the number of protons and electrons in the atom. Additionally, you can look up the charge of the ion and use it to find out the number of electrons.
Finding the number of electrons shouldn’t be difficult. With the help of this article, you can easily figure out how to find number of electrons quickly and accurately.
What is the easiest way to find the number of electrons?
If you’re a chemistry student, you’ll be familiar with the periodic table and the different elements that make it up. But did you know that you can use the periodic table to find out how many electrons are in each element? Knowing the number of electrons can be useful in a variety of scientific fields, from chemistry to physics. In this article, we’ll look at how to find the number of electrons in an atom, as well as how to use the periodic table to find the number of valence electrons and neutrons.
How to Find the Number of Electrons
Before learning further about how to find the number of electrons, let’s look at the following table of fundamental subatomic particles:
Atom | Protons | Neutrons | Electrons
— | — | — | —
Helium | 2 | 2 | 2
Neon | 10 | 10 | 10
Generally, the number of electrons—alongside with protons and neutrons—in an atom can be determined from a set of simple rules.
- The number of protons in the nucleus of the atom is equal to the atomic number (Z).
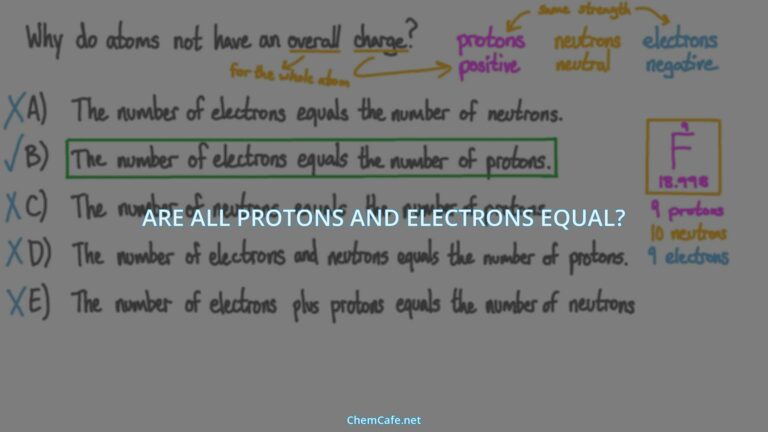
- The number of electrons in a neutral atom is equal to the number of protons.
- The mass number of the atom (M) is equal to the sum of the number of protons and neutrons in the nucleus.
- The number of neutrons is equal to the difference between the mass number of the atom (M) and the atomic number (Z).
How to Find Number of Valence Electrons
The valence electrons (VE) are the electrons in the outer shell of an atom. These are the electrons that are involved in the chemical reactions of an atom. The number of valence electrons determines the chemical properties of the atom. Generally, the number of valence electrons is equal to the group number of the element in the periodic table. For example, S-2 has 8 valence electrons.
How to Find the Number of Electrons in an Element Using Periodic Table
The easiest way to know how to find the number of electrons, neutrons, and protons for an element is to look at the element’s atomic number on the periodic table. That number is equal to the number of protons. To find the number of electrons in an element, you can use the following formula:
Number of electrons = Atomic number + Charge of the ion
For example, if you look at the atomic number for Oxygen (O) on the periodic table, it’s 8. If the oxygen atom has a charge of -2, then the number of electrons in the atom is 8 – 2 = 6.
You can also use the periodic table to find the number of neutrons in an atom. To do this, you need to subtract the atomic number from the mass number of the element. For example, if you look at the mass number of Oxygen (O), it’s 16. The number of neutrons in the oxygen atom is 16 – 8 = 8.
In conclusion, the periodic table can be used to find the number of electrons, protons, and neutrons in an atom. All you need to do is look up the element on the periodic table, determine its atomic number, and use the formula above to calculate the number of electrons. In addition, the number of valence electrons in an atom can be determined from the group number of the element. Knowing the number of electrons, protons, and neutrons in an atom can be useful in a variety of scientific fields.
How do you find the total number of electrons?
Finding the total number of electrons in an atom is essential for understanding the behavior of molecules and chemical reactions. The electrons are the particles that are responsible for the electrical properties of the atom and its interactions with other atoms and molecules.
The number of electrons in an atom is determined by the structure of its electron shells. The shells are layers of electrons arranged in concentric circles around the nucleus of the atom. Each shell can hold a maximum number of electrons, and each of these electrons is associated with a principal quantum number (n).
Finding the Atomic Number
The first step in finding the total number of electrons in an atom is to look up its atomic number on the periodic table. This number is located in the upper left-hand corner of the square that represents the element. The atomic number is the number of protons in the nucleus of the atom and is equal to the number of electrons.
Identifying the Ion Charge
The next step is to identify the charge of the ion. This is the number of electrons that have been added or removed from the atom to form the ion. It is written as a superscript to the right of the element on the periodic table.
Arranging Electrons into Shells and Subshells
To determine the total number of electrons, we must arrange them into shells and subshells. Each shell is associated with a principal quantum number (n), and the maximum number of electrons that can be accommodated in a shell is based on this number.
Table (PageIndex{2}) shows the maximum number of electrons that can be accommodated in each subshell. As an example, the first shell, which is closest to the nucleus and has the lowest-energy electrons, is shell 1. The maximum number of electrons that can be accommodated in shell 1 is 2n2, where ‘n’ is the shell number.
Calculating the Total Number of Electrons
To calculate the total number of electrons in an atom, add up the number of electrons in each of the shells and subshells. For example, if an atom has two electrons in shell 1 and six electrons in shell 2, the total number of electrons is 8.
The total number of electrons in an atom is an important piece of information that helps us understand the behavior of molecules and chemical reactions. By understanding the arrangement of electrons into shells and subshells, we can accurately calculate the total number of electrons.
How do you find the number of electrons?
Electrons are elementary particles that have a negative charge and are responsible for the chemical properties of atoms. Knowing the number of electrons in an atom is important for understanding its behavior. But how do you find the number of electrons?
Finding the Number of Protons
The first step in finding the number of electrons is to determine the number of protons in the atom. The number of protons in an atom is equal to the atomic number of the element. For example, the atomic number of oxygen is 8. This means that oxygen has 8 protons in its nucleus. Knowing the number of protons allows us to determine the number of electrons in the atom, because all atoms are electrically neutral. This means that the number of electrons must be equal to the number of protons.
What are Electron Configurations?
The electron configuration of an atom describes how the electrons are distributed in its atomic orbitals. Electron configurations of atoms follow a standard notation in which all electron-containing atomic subshells (with the number of electrons they hold written in superscript) are placed in a sequence. For example, the electron configuration of sodium is 1s22s22p63s1. However, the standard notation often yields lengthy electron configurations (especially for elements having a relatively large atomic number).
Table of Maximum Number of Electrons in Subshells
To find the number of electrons in a given atom, we need to look at the table of maximum number of electrons in subshells, as shown in Table (PageIndex{2}). This table shows us the maximum number of electrons that can occupy each subshell. For example, if an atom has an s subshell, it can hold a maximum of 2 electrons. Similarly, if an atom has a p subshell, it can hold a maximum of 6 electrons.
Using the Periodic Table
Once we know the number of protons and the maximum number of electrons in each subshell, the next step is to look at the periodic table. The periodic table will tell us the number of electrons in each subshell, based on the element’s atomic number. For example, if the atomic number of the element is 8, then it will have 2 electrons in its s subshell and 6 electrons in its p subshell.
In conclusion, finding the number of electrons in an atom is a relatively straightforward process. First, determine the number of protons in the atom. This is equal to the element’s atomic number. Then, use the table of maximum number of electrons in subshells to determine the maximum number of electrons that can occupy each subshell. Finally, use the periodic table to determine the number of electrons in each subshell, based on the element’s atomic number. With this information, you can now determine the number of electrons in the atom.
How do you count electrons in a molecule?
Atoms form molecules and compounds by sharing electrons to create chemical bonds. Understanding the nature of this bonding begins by knowing the number of electrons associated with each atom. With the information from a periodic table of the elements, and some straightforward arithmetic, you can calculate the number of electrons based on the chemical formula of a material.
Steps to Count Electrons in a Molecule
Step 1: Identify the chemical formula of the molecule. The formula is composed of the elemental symbols and numbers indicating how many atoms of each element are present. For example, KNO3 has one potassium atom, one nitrogen atom, and three oxygen atoms, and SO42- has one sulfur atom and four oxygen atoms.
Step 2: Look up the atomic number for each element in the periodic table and multiply the number of atoms of that element by the atomic number. This will give you the number of electrons that element contributes to the molecule. For example, the atomic number of potassium is 19, so the number of electrons contributed by the one potassium atom in KNO3 is 19 x 1 = 19. The atomic number of nitrogen is 7, so the number of electrons contributed by the one nitrogen atom in KNO3 is 7 x 1 = 7.
Step 3: For elements that have a negative charge, such as the oxygen atoms in KNO3, multiply the atomic number of the element by the charge on the element. In the case of KNO3, the oxygen atoms have a charge of -1, so the number of electrons contributed by the three oxygen atoms is 8 x -3 = -24.
Step 4: Add this value to the total from Step 3 to determine the total number of electrons in the molecule: 48 +2 = 50. Repeat for all elements in the molecule, then add up all the products to calculate the number of electrons. In the first example, the number of electrons in KNO3 equals (19 x 1) + (7 x 1) + (8 x 3) = 50. In the second example, the number of electrons in SO42- equals (16 x 1) + (8 x 4) = 48.
Tips for Counting Electrons in a Molecule
1. Be sure to remember the sign of the charge when calculating the number of electrons. A negative charge will result in a negative number of electrons, while a positive charge will result in a positive number of electrons.
2. The periodic table is a great resource for finding the atomic numbers of elements.
3. Remember to add all the values from Step 3 together to determine the total number of electrons in the molecule.
4. If you have trouble calculating the number of electrons, consult a periodic table or chemistry textbook for help.
Knowing how to count electrons in a molecule is an important part of understanding the nature of chemical bonds. With the information from a periodic table of the elements and some basic arithmetic, you can calculate the number of electrons in a molecule. This knowledge can be used to understand how atoms interact with each other, and how molecules are formed.
How do you find electrons per shell?
Atoms are composed of a nucleus that contains protons and neutrons, and electrons that occupy various shells around the nucleus. The number of electrons in each shell is determined by the atomic number of the element, and is a key factor in how the atom behaves. This article will explain how to find the number of electrons per shell and provide an overview of the Bohr model of the atom.
The Bohr Model
The Bohr model is a simplified representation of the atom that was proposed by Danish physicist Niels Bohr in 1913. According to this model, electrons occupy discrete energy levels, or shells, around the nucleus. The shells are labeled by the principal quantum number, n, with n = 1 representing the closest shell to the nucleus and n = 2 being the second closest, and so on.
The number of electrons in each shell is determined by the formula 2n2, where n is the principal quantum number. In other words, the first shell (n = 1) can hold 2 electrons, the second shell (n = 2) can hold 8 electrons, and so on. This rule applies to all atoms in the periodic table, regardless of their atomic number.
Valence Shells
The outermost shell of an atom is known as its valence shell. This shell determines how the atom behaves, as it is the electrons in this shell that are involved in chemical reactions. Typically, atoms will achieve a stable configuration when the outermost shell contains 8 electrons. This is known as the octet rule.
Atoms with fewer than 8 electrons in their valence shell will tend to gain electrons from other atoms in order to achieve a stable configuration. Atoms with more than 8 electrons in their valence shell will tend to lose electrons to other atoms. This behavior is what gives rise to chemical bonds between atoms.
The Limitations of the Bohr Model
It is important to note that the Bohr model is not an accurate representation of where electrons are at a specific moment in time. This model is still taught due to its simplicity, but it does not take into account the fact that electrons exist in a probability cloud, not in discrete shells.
Furthermore, the Bohr model does not account for the fact that electrons can occupy the same shell. In reality, the probability of finding two electrons in the same shell decreases as the number of electrons in the shell increases. This phenomenon is known as electron repulsion, and it is an important factor in determining the structure of molecules.
Conclusion
The number of electrons in each shell is determined by the atomic number of the element and the Bohr model of the atom. The outermost shell, or valence shell, determines how the atom behaves, as it is the electrons in this shell that are involved in chemical reactions. The Bohr model is not an accurate representation of an atom, but it is still taught due to its simplicity. Understanding the number of electrons in each shell and the limitations of the Bohr model is important for anyone studying chemistry or physics.
How do you determine the number of electrons?
When studying atoms, it’s important to understand how to determine the number of electrons. Electrons are negatively charged particles in the outermost shell of an atom and the number of electrons in an atom determines its chemical properties. Knowing how to calculate the number of electrons in an atom can help you understand its behavior in a chemical reaction.
Locating the Atomic Number
The first step in determining the number of electrons in an atom is to locate the element’s atomic number on the periodic table. The atomic number is the number of protons in the nucleus of the atom and is represented by the symbol Z. It is the upper left-hand corner of the square that contains the symbol for the element. For example, the atomic number of oxygen is 8.
Determining the Charge of the Ion
The next step is to determine the charge of the ion. This is written as a superscript to the right of the element on the periodic table. An ion with a positive charge has more protons than electrons, and an ion with a negative charge has more electrons than protons. For example, the charge of oxygen is -2, meaning it has 2 more electrons than protons.
Electron Shells and Subshells
The arrangement of electrons into shells and subshells is important to understanding the number of electrons in an atom. The first shell, closest to the nucleus and with the lowest-energy electrons, is shell 1. Each shell can contain a maximum number of electrons, as shown in Table (PageIndex{2}).
Table (PageIndex{2}): Number of Electrons
- Subshell Maximum Number of Electrons
- s 2
- p 6
- d 10
- f 14
What are Electron Configurations?
Electron configurations are descriptions of how electrons are distributed in an atom’s atomic orbitals. These configurations are written using a standard notation in which the electron-containing subshells and the number of electrons they hold are written in a sequence. For example, the electron configuration of sodium is 1s22s22p63s1.
Simplifying Electron Configurations
The standard notation for electron configurations can become quite lengthy, especially for elements with a relatively large atomic number. To simplify these configurations, the noble gas in the same period as the element can be used as a reference point. This means that instead of writing out the entire sequence of electron-containing subshells, you can simply write the noble gas symbol followed by the remaining electron-containing subshells. For example, the electron configuration of potassium can be simplified to [Ar]4s1.
In conclusion, determining the number of electrons in an atom is an important part of understanding its behavior in a chemical reaction. To calculate the number of electrons in an atom, start by looking up the element on the periodic table and locating its atomic number. Then, identify the charge of the ion, which will be written as a superscript to the right of the element. Finally, use the electron configuration to determine the number of electrons in the atom.


Leave a Comment