Understanding Rutherford’s Experiment
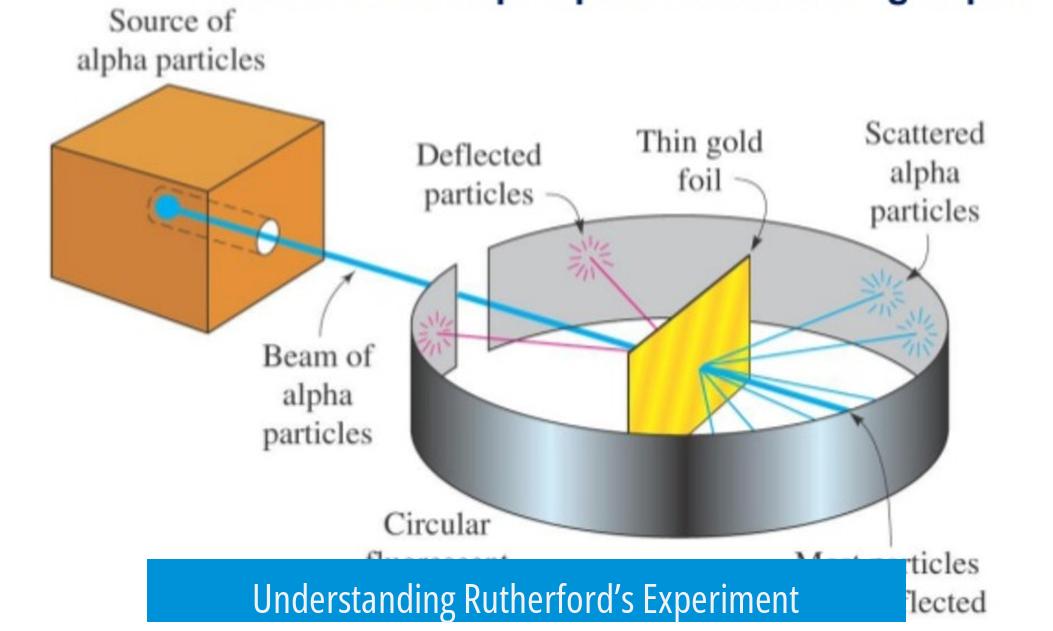
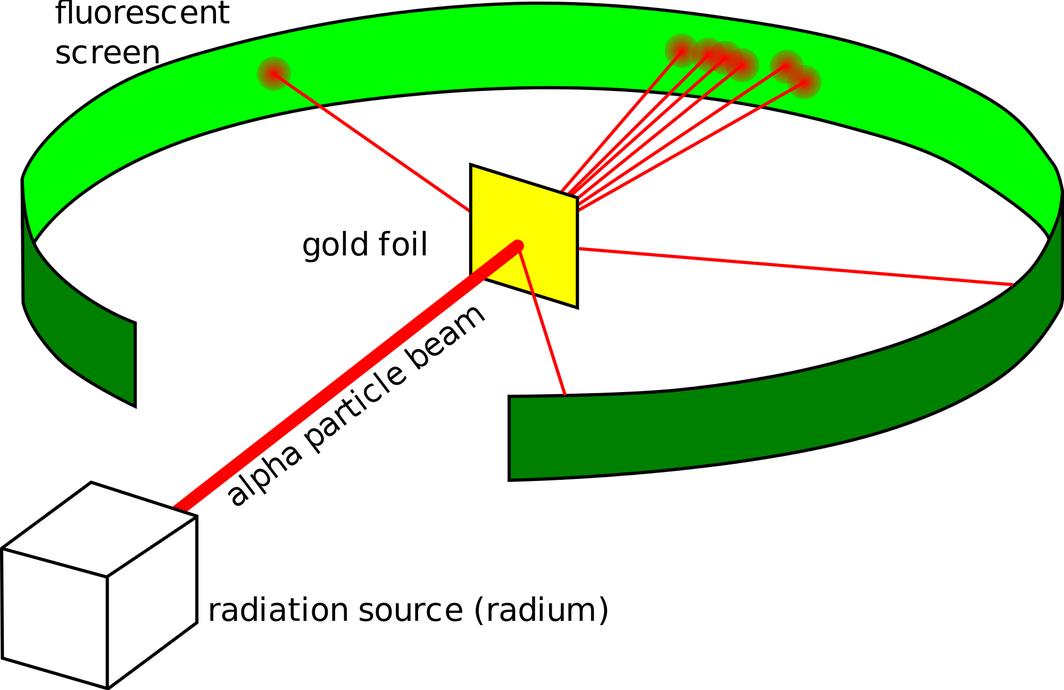
Rutherford’s experiment, conducted in 1909, aimed to investigate the structure of the atom by firing alpha particles through a thin sheet of gold foil and observing their deflection. The key revelation was that atoms comprise a small, dense nucleus surrounded by mostly empty space, overturning the previous atomic models.
Purpose and Concept of the Experiment
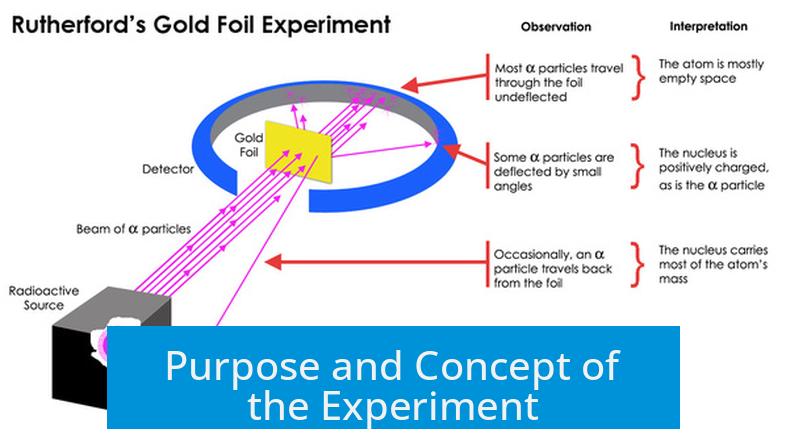
The goal was to understand how alpha particles interacted with solid matter. Alpha particles, which are helium nuclei composed of two protons and two neutrons, were chosen because their mass and charge allow them to collide effectively with atomic components.
Experimenters fired these alpha particles at a thin foil to analyze whether the particles would pass straight through or scatter. If atoms were as previously imagined—uniformly filled with electrons—particles would pass through with minor deflections. Instead, some alpha particles bounced back significantly, implying a different atomic structure.
Why Use Gold Foil?
- Material Characteristics: Gold can be beaten into extremely thin sheets, only a few atoms thick. This thinness was crucial to allow alpha particles to pass through without being absorbed entirely.
- Practical Choice: Gold’s ductility made it ideal for producing thin foil. It was a practical decision rather than chemical; other metals could not be formed as thinly without breaking.
Using thicker materials or metals less malleable than gold would have prevented alpha particles from passing through, making the experiment impossible.
Alpha Particles and Radiation Interaction
Alpha particles carry substantial energy but interact intensely with materials. They can generally be stopped by a sheet of paper. This contrasts with gamma rays, which penetrate most materials easily.
| Radiation Type | Penetration Ability | Analogy |
|---|---|---|
| Alpha Particles | Low – stopped by paper or skin | Truck moving into a forest, causes damage, stops quickly |
| Gamma Rays | High – pass through most materials | Bullet fired through a forest, passes largely unimpeded |
Prevailing Atomic Model Before the Experiment
Before Rutherford’s work, the atom was thought to be a “plum pudding” — a diffuse cloud of positive charge with electrons scattered within, much like raisins in a pudding. This model suggested electrons and positive charge were spread evenly without a defined center.
Scientists expected alpha particles to pass through this arrangement with only slight deflections caused by the distributed negative charge from electrons.
Unexpected Observations During the Experiment
Observations deviated from predictions:
- Most alpha particles passed through the foil with minimal deflection.
- A small fraction deflected at large angles, even bouncing backward in some cases.
These backward-deflections were startling. Only a small, dense, positively charged region could repel an alpha particle so strongly.
Discovering the Atomic Nucleus
Rutherford interpreted these deflections as evidence for a tiny nucleus at the atom’s center. This nucleus contained nearly all the atom’s mass and positive charge, explaining why only a few particles experienced strong repulsion.
Electrons orbited this nucleus, existing in vast empty space, which accounted for the high rate of alpha particles passing through largely undeflected.
How Alpha Particles Pass Through the Foil
Atoms are mostly empty space, with electrons occupying a large volume but having negligible mass compared to the nucleus. The alpha particle, heavy and fast, encounters few interactions in this space.
The thin gold foil increased the chance for an alpha particle to pass unimpeded. When deflections did occur, they pinpointed collisions with the dense nucleus.
Clarifications and Additional Insights
- Light does not play a role in this experiment. The interactions involve alpha particles, which are heavy charged particles, passing through matter.
- The outcome was revolutionary, reshaping atomic theory and laying the foundation for modern nuclear physics.
Summary of Key Points
- Rutherford fired alpha particles at thin gold foil to probe atomic structure.
- Gold foil was used for its ability to be made extremely thin.
- Alpha particles have high energy but interact strongly with matter, leading to limited penetration.
- Before the experiment, atoms were thought to have a diffuse positive charge.
- Some alpha particles deflected sharply, revealing a small, dense nucleus in atoms.
- Atoms are mostly empty space, with mass concentrated in the nucleus.
- The experiment disproved the plum pudding model and introduced the nuclear model of the atom.
Can Someone Explain Rutherford’s Experiment? Let’s Dive In!
Rutherford’s experiment fundamentally reshaped our understanding of atoms by revealing that atoms are mostly empty space with a tiny, dense nucleus at the center. This is the big headline, but how did he pull it off? Let’s unpack the experiment step-by-step, adding some fun and clarity along the way.
Picture yourself trying to explore a fog cloud without stepping inside it. You can’t just walk around to investigate because the fog is thick. So what do you do? You fire cannonballs through different spots of the cloud. You watch where the balls go freely and where they bounce back. This simple analogy gets to the heart of Rutherford’s clever approach to peering inside an atom without actually breaking it open.
The Bold Idea: Shooting Alpha Particles Through Matter
Back in 1909, scientists believed the atom was like a plum pudding—a big blob of positive charge sprinkled randomly with tiny electrons. This was J.J. Thomson’s model, and honestly, it wasn’t very exciting or accurate.
Rutherford asked, “What if we fire alpha particles, which are helium nuclei, at a super-thin sheet of metal and watch what happens?” Why alpha particles? Well, they’re solid in a way—made of two protons and two neutrons—so they can smash into things and give a clear hint of what’s inside atoms.
This was no cloaked magic bullet situation. They knew alpha particles pack a punch but also don’t travel far, making them perfect probes to test atomic structure.
Gold Foil: The Perfect Target
Why gold? Because it can be hammered into sheets just a few atoms thick. This tiny, ultra-thin foil behaves almost like a delicate veil, allowing alpha particles to pass through it rather than get stuck like in thicker metals. Rutherford’s team chose it purely for practical reasons—not because gold is chemically special, but because it’s incredibly ductile and thin sheets can be made. Try that with steel, and you’ll have a chunk, not a film.
Expectations Before the Test
The prevailing theory suggested alpha particles would blast straight through the atoms with minor nudges, given atoms were thought to be a diffuse mix of charges. No one expected much drama because the positive charge was assumed spread out uniformly, so the particles wouldn’t notice any “hard bumps.”
Imagine tossing a tiny marble into a cloud of cotton balls—you’d expect it to pass with just some gentle resistance.
The Big Surprise: Particles Scattered Like a Pinball Machine
The data stunned Rutherford’s team. While many alpha particles did sail right through with small deflections, a surprising few bounced back almost directly toward the source. It was like firing a cannonball, only to have it boomerang back at you! This was unexpected, shocking the scientific community.
How to make sense of this? Only one conclusion fit: the atom contains a very small, dense core that is positively charged, deflecting the alpha particles. The rest of the atom is mostly empty space, allowing far-away particles to fly straight through.
Modern Atomic View Backed Up
Today, we know that the nucleus holds nearly all the mass of the atom, packed with protons and neutrons, while electrons buzz around it, light and mostly charge without mass blocking the way. This matches perfectly what Rutherford observed. Since the experiment uses a super-thin gold foil, most alpha particles don’t hit the dense nucleus and keep moving forward.
Think of the alpha particle like a heavy truck barreling down a narrow road filled mostly with empty space—the chance of hitting a solid obstacle is low, but when it does, wow does it react.
Alpha Particles Aren’t Just Another Kind of Radiation
It’s easy to confuse all radiation, but alpha particles are unique. They’re energetic but interact strongly with matter. They can’t penetrate far—blocked by just a piece of paper. Contrast that with gamma rays, which behave like tiny bullets zooming through forests of atoms without hardly a scratch. This difference makes alphas ideal for pinpointing dense spots inside atoms.
Clearing Up Misconceptions
One common confusion: some people think light was involved in the experiment. Nope! No light, no photons—just alpha particles crashing through gold foil, simple and pure.
Funny side note: Rutherford’s name often sparks chuckles among physics students because their teachers affectionately called him “pimp daddy Rutherford” for his gold foil experiment. Tricky nickname, but it does highlight how groundbreaking his work was!
Why Does This Matter Today?
Rutherford’s experiment is a staple example of how careful observation can shatter long-held beliefs. It shows the power of simple ideas executed well. Plus, it paved the way for the nuclear age—from understanding atomic energy to medical imaging.
So next time you wonder about atoms, remember those alpha particles zipping through gold foil, revealing secrets so tiny they shake the foundations of science.
In Summary
- Rutherford fired alpha particles, solid helium nuclei, through an ultra-thin gold foil to probe atomic structure.
- They expected slight deflections but found some particles bounced back sharply, implying something dense inside atoms.
- This led to discovering the nucleus: a tiny, massive, positively charged core surrounded by mostly empty space.
- Gold foil was chosen for its ultra-thinness, letting particles pass through rather than get trapped.
- Alpha particles are unique probes because they carry a lot of mass and charge but don’t penetrate far, making them perfect for hitting dense atomic cores.
Rutherford’s experiment shines as a brilliant example of curiosity meeting experimental prowess. It answers: “What’s inside an atom?” with a crystal-clear “mostly empty space and a dense nucleus.” Isn’t science just dazzling?
What was the main goal of Rutherford’s experiment?
The goal was to fire alpha particles through solid matter and observe their path. This helped understand the structure of atoms by seeing how particles interacted with the material.
Why did Rutherford use gold foil in his experiment?
Gold can be rolled into extremely thin sheets, only a few atoms thick. This thin foil allowed alpha particles to pass through or be deflected, making it ideal for studying atomic structure.
What surprising observation did Rutherford make about alpha particles?
Some alpha particles passed through with slight deflections, but others bounced back almost entirely. This was unexpected and suggested the presence of a small, dense nucleus in atoms.
How did Rutherford’s findings change the atomic model?
He discovered that atoms are mostly empty space with a small, dense nucleus at the center. This disproved the earlier “plum pudding” model, establishing the nuclear model of the atom.
Why do alpha particles behave differently than gamma rays in materials?
Alpha particles are heavy and interact strongly, so they can be stopped by thin materials like paper. Gamma rays are more penetrating and pass through materials more easily.




Leave a Comment