Understanding the Canonical Order of Capping, Splicing, and Polyadenylation in mRNA Processing
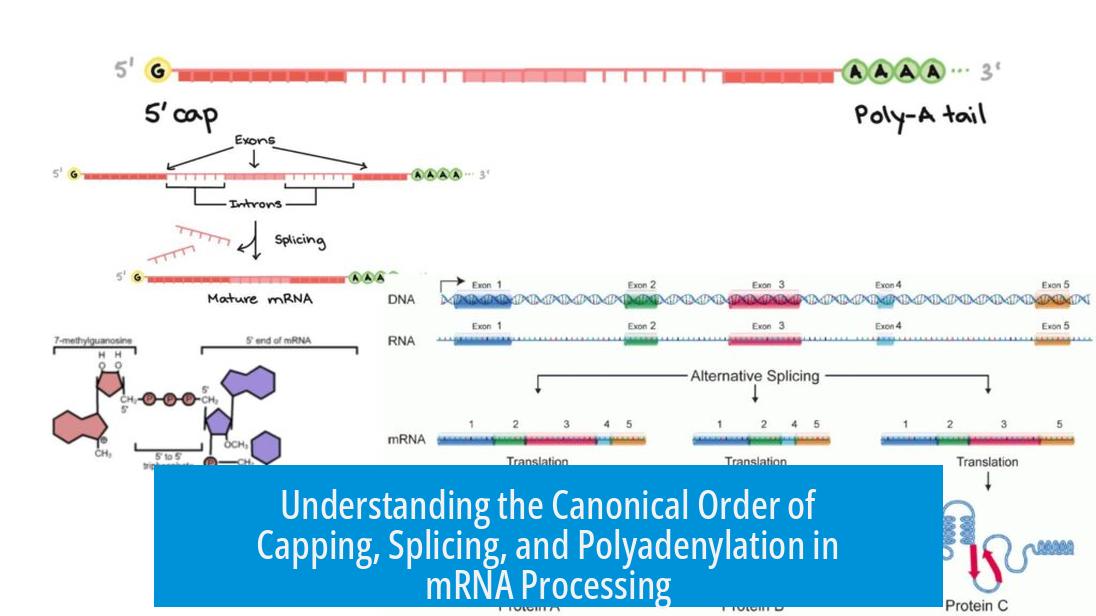
The canonical order of capping, splicing, and polyadenylation in eukaryotic pre-mRNA processing typically starts with capping at the 5′ end, followed by splicing of introns, and finishes with polyadenylation at the 3′ end after transcription completes.
This sequence reflects coordinated molecular events that modify the newly synthesized RNA to produce mature messenger RNA ready for translation. Each step is tightly regulated and linked to transcription by RNA polymerase II.

1. Cotranscriptional Nature of RNA Processing
RNA processing initiates while the RNA is still being synthesized, a phenomenon termed cotranscriptional processing. The newly formed 5′ end of the pre-mRNA emerges from RNA polymerase II and is immediately modified.
The enzyme complex that adds the 5′ cap associates with RNA polymerase II from the beginning of transcription. This early engagement ensures rapid capping, protecting the nascent RNA.
2. Capping: The First Modification

A 7-methylguanosine cap attaches to the 5′ end of the pre-mRNA shortly after transcription initiation. This cap prevents degradation and plays a key role in recruiting the ribosome for protein synthesis. The capping machinery operates almost simultaneously with the emerging RNA strand.
3. The Role of RNA Polymerase II in Coordination

The C-terminal domain (CTD) of RNA polymerase II serves as a molecular platform. It recruits various RNA processing factors at different stages of transcription. While elongation proceeds, factors responsible for splicing and 3′ end formation attach to the CTD and interact with the growing RNA.
4. Splicing Occurs Co- and Post-Transcriptionally

As the splice sites within the pre-mRNA appear, the spliceosome assembles on these regions to remove introns. Evidence shows splicing can occur while transcription is ongoing (co-transcriptionally), but it also continues after the RNA polymerase has passed the gene region (post-transcriptionally).
The assembly of the spliceosome depends on the immediate recognition of splice sites. This process ensures that the RNA is properly excised and prepared for final maturation.
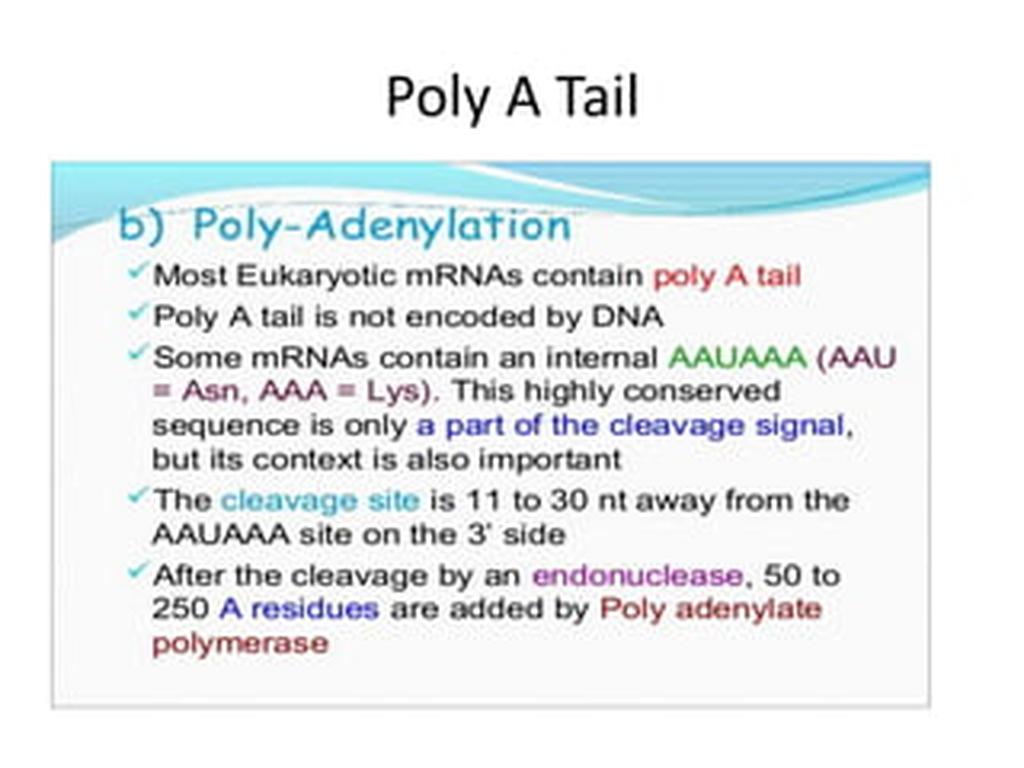
5. Polyadenylation Follows Transcription Termination
Once RNA polymerase II transcribes the gene’s end, the 3′ processing machinery is recruited to the CTD. This machinery cleaves the RNA downstream of specific signals and adds a poly(A) tail to the 3′ end of the pre-mRNA.

The poly(A) tail stabilizes the mature RNA, facilitates its export to the cytoplasm, and supports translational initiation by recruiting poly(A)-binding proteins.
6. Summary of the Canonical Order
| Step | Description | Timing |
|---|---|---|
| Capping | 7-methylguanosine cap addition at the 5′ end. | Immediately as RNA emerges from RNA polymerase II. |
| Splicing | Intron removal via spliceosome assembly, occurs co- and post-transcriptionally. | As soon as splice sites are transcribed and continue afterwards. |
| Polyadenylation | Addition of poly(A) tail following 3′ end cleavage. | After transcription termination and 3′ end cleavage. |
7. Crosstalk and Interaction Between Processes
The boundaries between splicing and polyadenylation are not rigid. Some splicing reactions can occur after the poly(A) tail is added. Molecular interactions link spliceosomal components with the 3′ end processing machinery to coordinate final RNA maturation.

This coordination optimizes RNA stability, export, and translation efficiency.
8. Variability and Context Dependency
The timing of splicing relative to polyadenylation can differ by gene and cell type. Regulatory elements, RNA-binding proteins, and RNA modifications modulate this interplay. Ongoing research investigates how these variations affect gene expression.
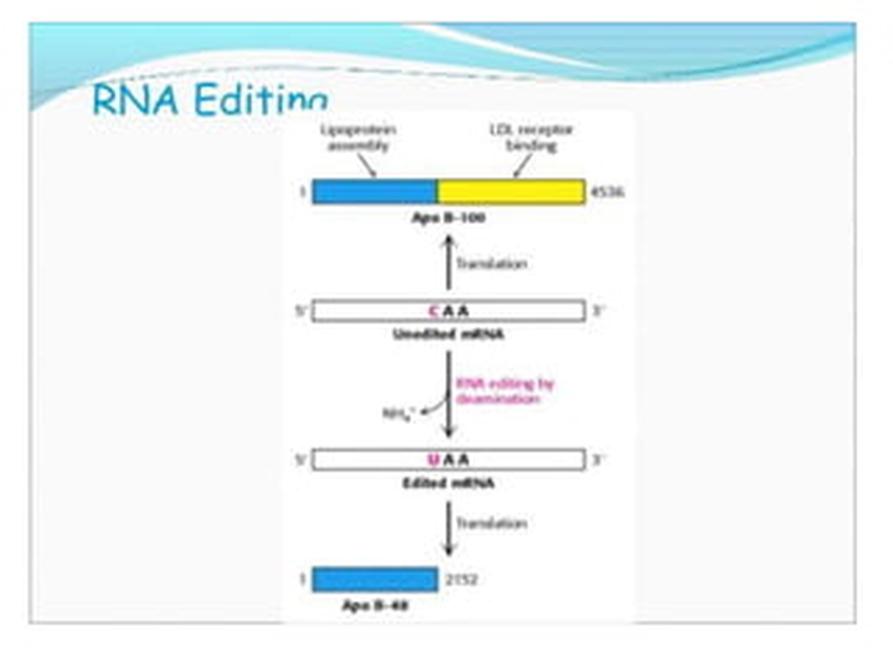
RNA editing and other modifications influence splicing and polyadenylation events, showing the complexity of RNA maturation dynamics.
9. Mechanistic Insights from Research
- RNA polymerase II’s CTD serves as a hub recruiting enzymes for capping, splicing, and 3′ end processing (Shine et al., 2024; Merens et al., 2024).
- Spliceosome assembly occurs almost immediately at transcribed splice sites (Choquet et al., 2025).
- Polyadenylation follows cleavage of the 3′ end after transcription finishes (Kaida, 2016).
- Reciprocal regulation between splicing and 3′ end processing ensures precise mRNA maturation (Reimer et al., 2021).
10. Practical Implications
Understanding this canonical order is essential for interpreting gene expression regulation and RNA biology. Aberrations in any of these steps can cause defective mRNA and contribute to disease.
Biotechnological applications, such as mRNA therapeutics, depend on manipulating these RNA processing steps effectively.
Key Takeaways
- Capping is the earliest step, adding a protective 5′ cap almost immediately as RNA is transcribed.
- Splicing occurs during and after transcription, removing introns from the pre-mRNA.
- Polyadenylation happens last, adding a poly(A) tail after transcription termination and 3′ end processing.
- RNA polymerase II’s C-terminal domain coordinates processing events by recruiting necessary enzymes.
- Splicing and polyadenylation interact closely and can influence each other’s efficiency.
- The precise timing can vary depending on the gene and cell context.
What is the Canonical Order of Capping, Splicing, and Polyadenylation?
The canonical order of capping, splicing, and polyadenylation during eukaryotic mRNA processing generally follows a neat sequence: first capping, then splicing, and finally polyadenylation. This order mirrors how the cell machinery processes the RNA molecule as it is being transcribed and ensures proper maturation for translation. But let’s dissect this biological ballet step-by-step to see why the order matters and what happens in practice.
If you’ve ever wondered how your DNA’s messages transform into reliable, working mRNAs, you’re about to get some insights into the choreography of these molecular events.
Capping: The Very First Act
As soon as RNA polymerase II starts transcribing a gene, a special hat gets put on the emerging RNA strand. This is the 7-methylguanosine cap, added to the 5′ end of the nascent pre-mRNA. This capping is no joke — it happens immediately as the RNA emerges from the polymerase.
Why so quick? The cap protects the fragile new RNA from enzymatic destruction. It also signals that this RNA is legitimate and helps ribosomes find the RNA later during translation. The proteins responsible for capping actually hitch a ride on RNA polymerase’s tail and apply the cap co-transcriptionally, almost in real-time.
Think of capping like putting a quality seal on a cinematic script right when the first page is written.
Splicing: The Middle Editor
Once the RNA polymerase moves along the DNA, it’s not just writing straight text — it’s writing several segments, some of which (introns) aren’t meant to be in the final script. Here enters splicing. The spliceosome assembles right after the splice sites appear on the pre-mRNA.
The cool part? Splicing kicks in co-transcriptionally (while the RNA is still being made) but can also continue after transcription ends. The cell’s machinery starts trimming introns as soon as possible but might finish editing the message a bit later, before the mRNA leaves the nucleus.
Splicing’s timing is somewhat flexible. Sometimes it takes place before the polyadenylation step, but depending on the gene, it can occur after or even overlap.
Polyadenylation: The Grand Finale
After RNA polymerase II transcribes the full length of the gene, the cell switches gears for its last act — polyadenylation. This involves adding a string of adenines called the poly(A) tail to the 3′ end of the pre-mRNA.
The poly(A) tail is more than decoration; it protects the mRNA against degradation and assists in exporting the message out of the nucleus. It also plays a key role in translation initiation. But here’s the clincher: this tail gets added only after the pre-mRNA is cleaved at its 3′ end, marking the conclusion of transcription.
Unlike capping, which is lightning fast and immediate, polyadenylation is distinctly post-transcriptional, happening when the gene is completely copied.
A Symphony Conducted by RNA Polymerase II
RNA polymerase II isn’t just a passive scribe. It is more like a maestro who coordinates the entire processing orchestra — recruiting capping enzymes at the start, splicing factors during elongation, and then the polyadenylation machinery near the end.
The large C-terminal tail of RNA Pol II acts as a platform to pass the right tools to the pre-mRNA at the right time. This coordination means the cell can efficiently process thousands of RNA molecules while avoiding costly errors.
Are Splicing and Polyadenylation Always Strictly Ordered? Not Exactly.
While textbooks often present the order as capping → splicing → polyadenylation, reality is more nuanced. Some splicing events can happen post-polyadenylation, and certain genes show intricate cross-talk between splicing and polyadenylation machinery.
For instance, various studies (Reimer et al., 2021; Kaida, 2016) report that specific intron removal may influence how the cleavage and polyadenylation complex operates. This interplay implies that while the general sequence holds, exceptions exist, often depending on the gene type or cell environment.
It begs the question: could there be regulatory advantages to this flexibility? Most likely yes. It lets the cell fine-tune expression and respond dynamically to different needs.
Why Should You Care About This Order?
The processing order is crucial for mRNA stability, translation efficiency, and ultimately protein production. Missteps can lead to faulty proteins or diseases.
For example, improper capping can cause rapid degradation. Incomplete or incorrect splicing can yield nonfunctional proteins or even trigger diseases like spinal muscular atrophy or certain cancers. Faults in polyadenylation may degrade mRNA prematurely or interfere with translation.
Understanding this order is vital in biotechnology and medicine. Therapeutic strategies, like antisense oligonucleotides or RNA vaccines, hinge on manipulating these processing steps.
Summary Table: Canonical Order of mRNA Processing
| Step | Description | Timing |
|---|---|---|
| Capping | Addition of 7-methylguanosine cap at the 5′ end during early transcription | Immediately as RNA emerges |
| Splicing | Removal of introns as splice sites appear; spliceosome assembles co-/post-transcriptionally | Co-transcriptional and post-transcriptional |
| Polyadenylation | Addition of poly(A) tail after 3′ end cleavage near transcription termination | Post-transcriptional |
In Conclusion
The canonical order — capping first, splicing second, and polyadenylation last — mirrors how pre-mRNA matures into a stable, translation-ready transcript. This sequence is orchestrated beautifully by RNA polymerase II and its associated processing complexes to maintain gene expression fidelity.
Yet, the order is more of a guideline than a rigid rule. The interplay and timing of these processes can shift with different genes and cell types, reflecting the complexity of gene regulation.
Next time you read about gene expression, remember: this isn’t just a linear tale, but a finely tuned performance with actors arriving just in time to make the perfect messenger RNA.
Curious to see how evolving research might further refine this “canonical” pathway? Stay tuned, because molecular biology’s script is still being written.




Leave a Comment