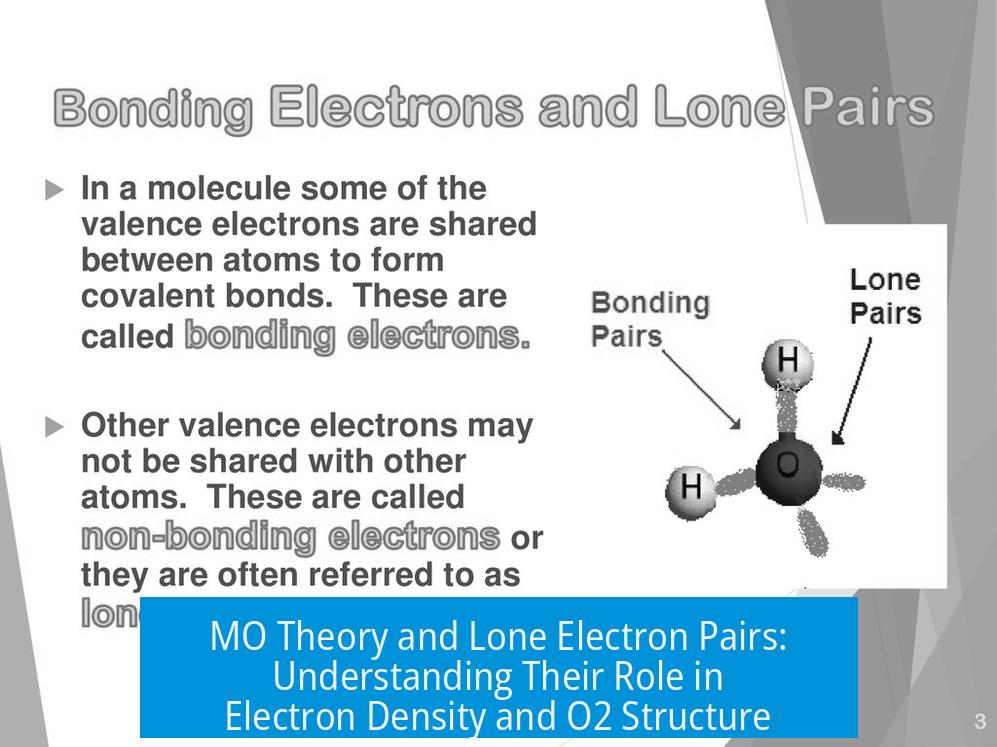
MO Theory and Lone Electron Pairs
Lone electron pairs are found in nonbonding molecular orbitals, not in bonding or antibonding orbitals. This distinction defines their unique role in molecular structure. Unlike bonding electrons that hold atoms together or antibonding electrons that weaken bonds, lone pairs occupy orbitals that do not directly participate in bonding.
Lone Pairs in Molecular Orbital Theory
Molecular Orbital (MO) theory classifies orbitals as bonding, antibonding, or nonbonding. Lone pairs are electrons located in nonbonding orbitals. These orbitals are fully occupied and isolated from the bonding framework of the molecule.
- Nonbonding orbitals neither stabilize nor destabilize the molecule significantly.
- Lone pairs influence molecular shape and reactivity through electron density localized on individual atoms.
- This placement contrasts with Lewis structures, where lone pairs are simply shown as paired dots on atoms.
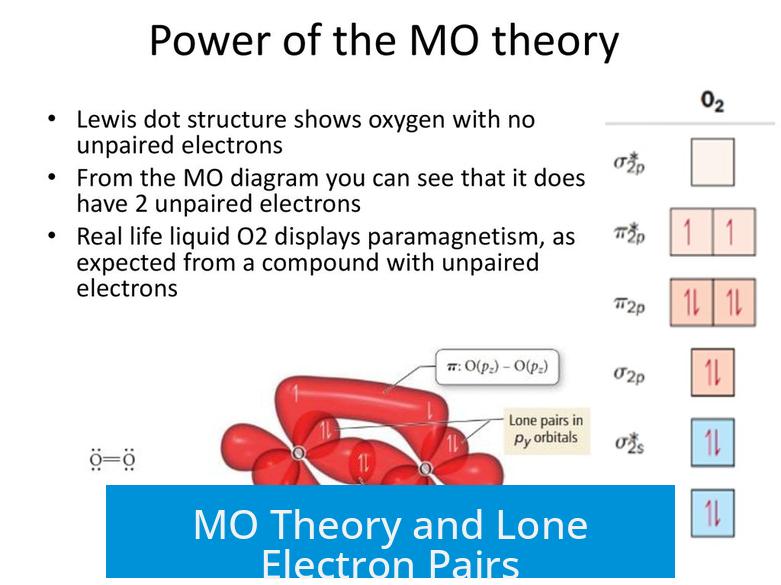
Limitations of Lewis Structures in Describing O2
The classic Lewis depiction of oxygen (O2) falls short when explaining its electronic structure. In Lewis structures, electrons appear strictly as bonding pairs or lone pairs. However, for O2, all electrons fit into bonding or antibonding molecular orbitals, with no true lone (nonbonding) pairs present.
Moreover, the Lewis model cannot explain the paramagnetism of O2. Experimentally, O2 is paramagnetic, meaning it has unpaired electrons. The MO diagram reveals two unpaired electrons reside in antibonding π* orbitals, confirming this magnetic property.
Electron Density Distribution in O2 from MO Theory
MO diagrams also clarify electron density distribution in O2. Filled antibonding orbitals place significant electron density at the molecule’s ends, away from the internuclear region.
| Aspect | MO Theory | Lewis Structure |
|---|---|---|
| Electron Location | Electron density found in bonding and antibonding orbitals; antibonding electrons localize at the ends. | Electrons shown as either bonding pairs or lone pairs on atoms; no clear spatial electron density info. |
| Electron Pairing | Presence of unpaired electrons explains paramagnetism | All electrons paired; fails to predict paramagnetism. |
Though the Lewis structure crudely hints at electron density not “in the bond,” it does not convey the complexity that MO theory reveals. MO theory captures the true electronic behavior within molecules like O2.
Key Takeaways
- Lone electron pairs occupy nonbonding orbitals in MO theory, distinct from bonding and antibonding orbitals.
- Lewis structures do not accurately describe the bonding in O2, missing unpaired electrons and nonbonding electrons’ behavior.
- MO diagrams explain O2 paramagnetism through unpaired electrons in antibonding orbitals.
- Electron density distribution in O2 differs from Lewis model predictions, shown clearly by MO theory.
Understanding MO-Theory and Lone Electron Pairs: A Fresh Perspective
If you ever wonder where those pesky lone electron pairs hide in molecular structures, the Molecular Orbital (MO) theory has the scoop: lone pairs reside in nonbonding orbitals, distinct from bonding or antibonding ones. This detail is a game-changer. Why? Because it explains why lone pairs don’t take part in bonding but still influence molecular geometry and reactivity. Ready to dive deeper?
Let’s unravel what MO theory says about lone pairs, and how it brings clarity where traditional Lewis structures sometimes trip up—especially in a famous molecule like O2.
Why Lone Electron Pairs Are Not Just “Leftover” Electrons
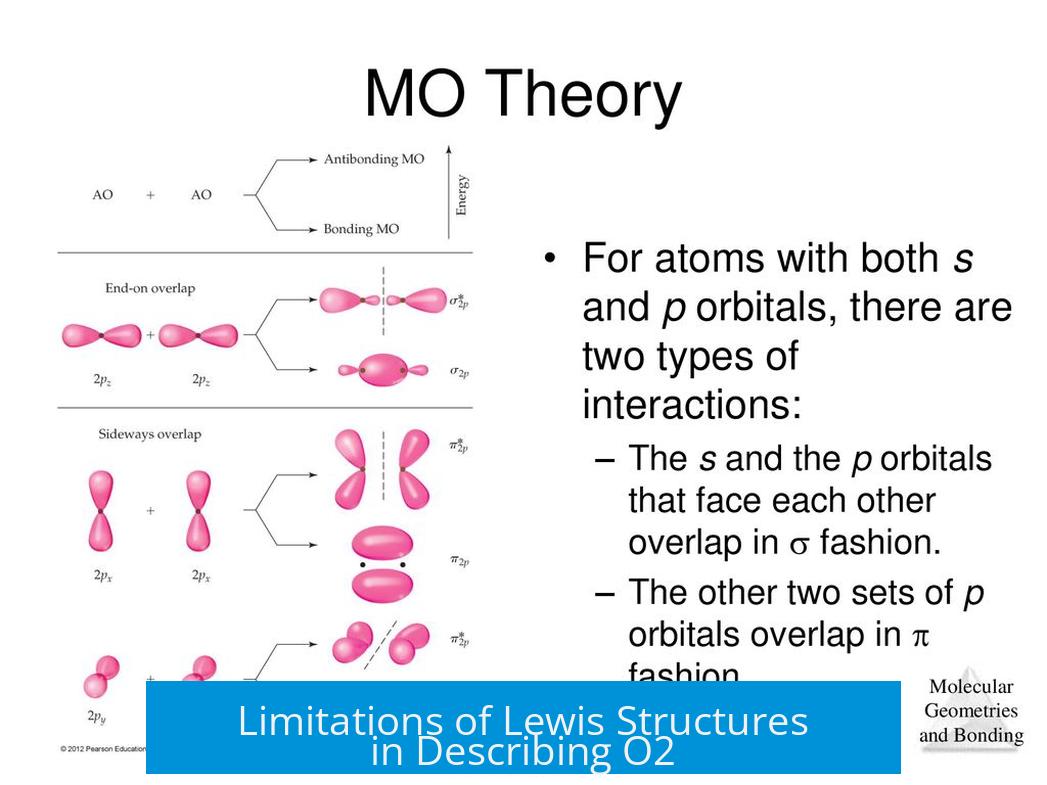
Lewis structures often portray lone pairs as “leftover dots” hanging around an atom, giving us a rough idea of molecular shape. But reality is a bit more nuanced. MO theory introduces the concept of orbitals that can be bonding, antibonding, or nonbonding.
Lone pairs occupy nonbonding orbitals. This means these electrons neither help glue atoms together (bonding) nor weaken bonds by out-of-phase interactions (antibonding). Instead, they hold their own cozy spots in the molecule’s electron cloud, keeping to themselves. This classification explains why lone pairs impact molecular geometry: they push bonding pairs around.
In everyday terms, if bonding pairs are busy partners shaking hands, lone pairs are like the introverts standing nearby, influencing the scene without joining the handshake.
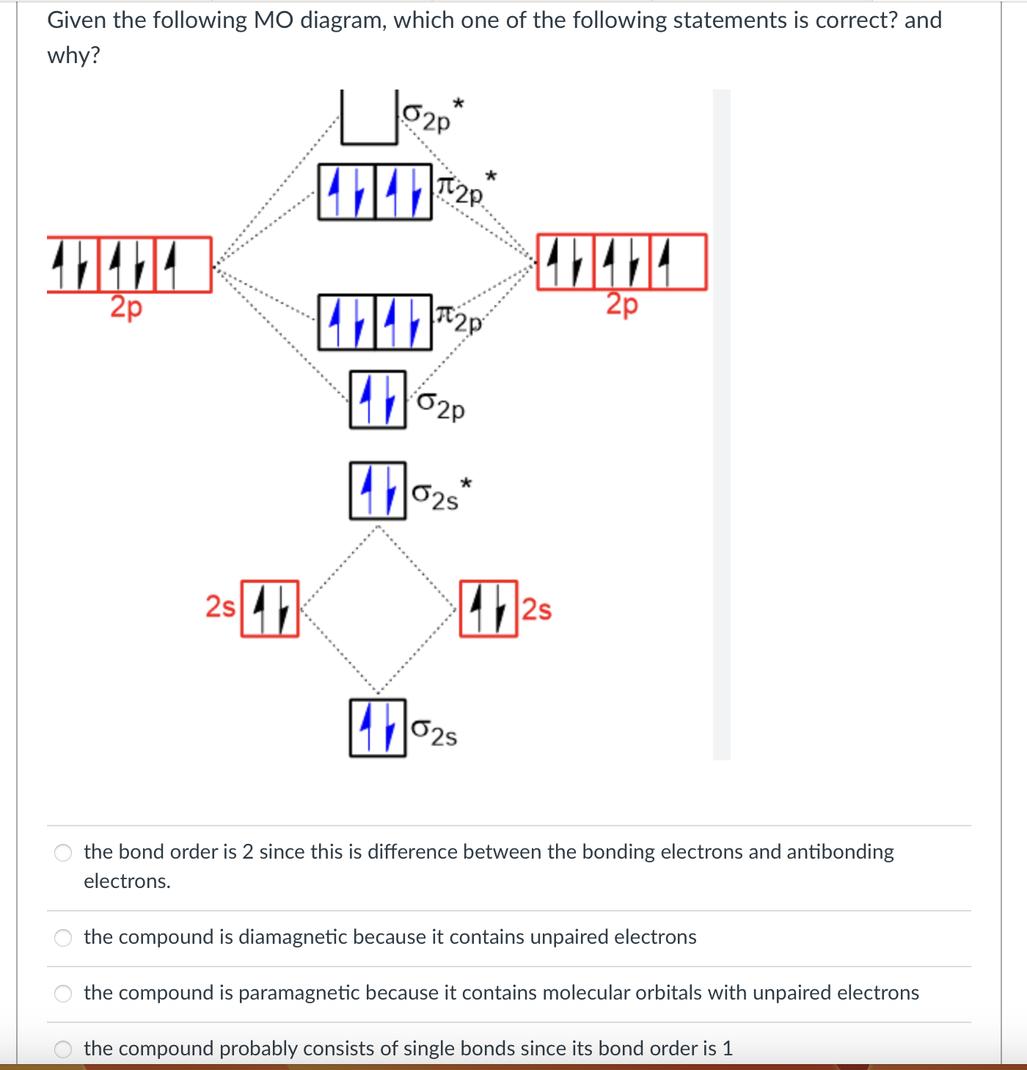
When Lewis Structures Meet Their Match: The Case of O2
Ever tried to explain oxygen’s behavior using just Lewis structures? You’ll quickly notice something is off. Here’s why:
- Lewis structures for O2 show all electrons paired in bonds or lone pairs.
- But experimentally, O2 is paramagnetic, meaning it has unpaired electrons.
This is exactly where MO theory shines. It reveals that O2 doesn’t have any nonbonding orbitals full of lone pairs. Instead, electrons occupy antibonding orbitals, some unpaired, which explains the paramagnetism.
Simply put, Lewis structures give a neat, tidy picture but miss the molecular truth. They ignore subtle electron behavior, like those wandering unpaired electrons that make O2 magnetically interesting.
How MO Diagrams Change Our Picture of Electron Density in O2
Ever squinted at a Lewis structure wondering where exactly electrons hang out? MO theory pulls back the curtain. It shows that because many antibonding orbitals in O2 are filled, electron density isn’t concentrated between the atoms as Lewis might suggest. Instead, a lot of electron density sits on the ends of the molecule.
This affects the molecule’s shape, reactivity, and physical properties. Electrons at the ends reduce bond strength and lengthen bonds, nuances missed by simpler models.
You might be tempted to say Lewis structures somewhat hint at this by showing electrons “outside” the bonds, but that’s not their main goal. Lewis forms focus on bond connections, not electron density distributions. MO theory nails these details, guiding chemists to better predict molecule behavior.
Practical Insights: Why Knowing About Lone Pairs in MO Theory Matters
Getting these distinctions right isn’t just academic nitpicking. It has real-world payoffs:
- Predicting molecular geometry and reactivity: Lone pairs in nonbonding orbitals push bonding electrons around, explaining shapes and bond angles more accurately than Lewis structures.
- Understanding magnetic properties: For O2, MO theory explains paramagnetism, crucial for fields like material science and biochemistry.
- Improved interpretation of spectroscopy and reactions: Knowing where electrons reside guides chemists when designing catalysts or drugs.
Can We Use MO Theory to Improve Everyday Chemistry Learning?
Absolutely. While Lewis symbols are great teaching tools, supplementing them with MO theory concepts creates a richer understanding. For instance, drawing attention to where lone pairs live helps students appreciate why molecules behave as they do.
So next time you sketch chlorine or water molecules, remember: those lone pairs aren’t just dots. They’re electrons chilling in nonbonding orbitals, silently shaping chemistry.
Final Thoughts: The Big Picture on MO Theory and Lone Pairs
MO theory doesn’t just add complexity. Instead, it provides clarity, revealing the hidden dance of electrons in molecules. Lone pairs are no mere bystanders—they occupy distinct, nonbonding orbitals that influence molecular traits quietly but decisively.
And when Lewis structures hit a wall, like predicting O2’s magnetism, MO theory steps in as the microscope revealing the true electron arrangement. It’s a reminder that chemistry often requires more than neat lines on paper—it demands thinking about electrons as clouds, waves, and probabilities.
Ready to embrace MO theory in your chemistry journey? Understanding lone electron pairs better is a major step forward. It’s the difference between seeing molecules in black and white versus living color—and that’s a pretty bright upgrade.
What type of orbitals do lone electron pairs occupy in MO theory?
Lone pairs reside in nonbonding orbitals. These orbitals neither form bonds nor are antibonding. They remain filled but do not affect bonding directly.
Why can’t Lewis structures accurately describe the bonding in O2?
Lewis structures lack nonbonding orbitals for O2. They show all electrons either in bonding or antibonding orbitals. This misses key features like unpaired electrons.
How does MO theory explain the paramagnetism of oxygen molecules?
MO theory reveals unpaired electrons in O2. Their presence matches the molecule’s paramagnetic behavior, something Lewis structures fail to predict.
What does the electron density distribution in O2’s MO diagram show?
The diagram highlights filled antibonding orbitals. This results in electron density concentrated at the molecule’s ends, not just between the atoms.




Leave a Comment