Understanding the Point Group D3h
The point group D3h describes molecules with trigonal planar geometry exhibiting specific rotational and reflection symmetry elements, such as BH3. This group includes a principal threefold rotational axis (C3) perpendicular to the molecular plane and distinct symmetry operations arising from multiple rotation angles around this axis.
Molecular Geometry and Example
BH3 exemplifies the D3h point group due to its trigonal planar shape. In this molecule, the boron atom sits at the center with three hydrogen atoms arranged evenly in a plane around it. This spatial arrangement results in characteristic symmetry elements defining the group.
C3 Axis Orientation
The principal symmetry axis, labeled C3, passes through the boron atom. It is orthogonal, or perpendicular, to the plane formed by the three hydrogen atoms. This axis allows for rotations that leave the molecule essentially unchanged.
Distinct C3 Rotational Operations

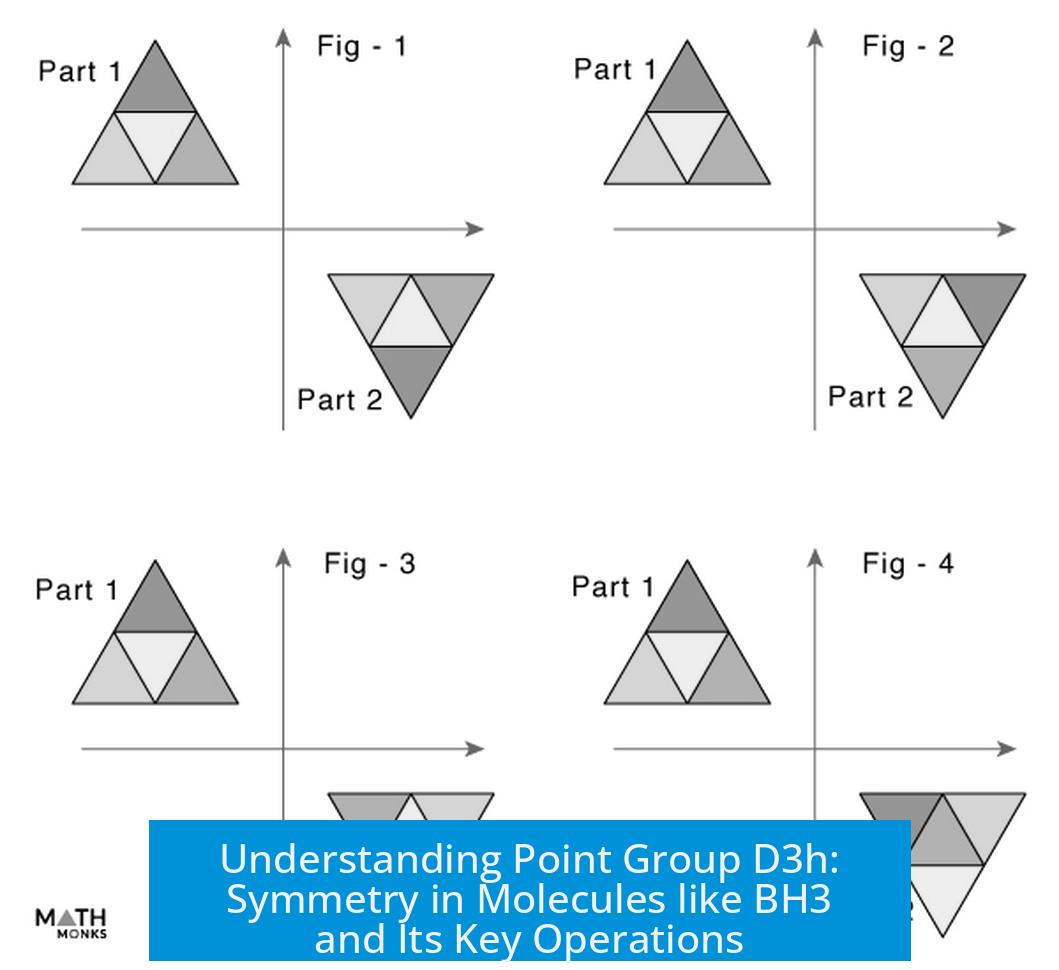
- C3: Rotation of 120° around the C3 axis.
- C32 (or C3′): Rotation of 240° (120° twice), often interpreted as a rotation in the opposite direction.
Both rotations move the hydrogen atoms into new but symmetry-equivalent positions, creating two unique C3 operations within the group. Each alters the position of hydrogens differently, although they are symmetry-related.
Counting Symmetry Operations in D3h

The existence of these two distinct rotational operations around the single C3 axis means the D3h point group contains 2 C3 operations. Their uniqueness contributes to the molecule’s overall symmetry characterization.
Visual and Experimental Confirmation
Constructing physical models or accessing online resources like symotter.org allows one to observe these symmetry operations firsthand. Software tools such as the “space group visualizer” provide interactive ways to explore rotational axes, mirror planes, and other symmetry elements for D3h and related groups.
One Axis, Two Distinct Operations
Although only a single geometric C3 axis exists, rotating clockwise or counterclockwise defines two different and distinct symmetry operations. This duality is fundamental when analyzing molecular symmetry in D3h molecules.
Key Takeaways
- D3h point group applies to trigonal planar molecules like BH3.
- The principal C3 axis is perpendicular to the molecular plane through the central atom.
- Two unique C3 operations (120° and 240° rotations) define distinct symmetry elements.
- Both clockwise and counterclockwise rotations represent different symmetry operations despite a single geometric axis.
- Experimental models and visualization software aid in understanding D3h symmetry.
Point Group D3h: Unlocking the Symmetry Magic in Molecules Like BH3
What is the Point Group D3h exactly? Simply put, it’s a set of symmetrical operations that perfectly describe molecules like BH3, which sport a trigonal planar shape. The star of this show is the B-H molecule BH3, a textbook example of D3h symmetry. So, why should you care about this symmetry group? Because understanding it reveals how atoms dance around in a molecule, defining its properties and behaviors. Let’s unpack the details and have a little fun while we’re at it.
Meet BH3: The D3h Poster Child
Imagine BH3 like a tiny molecular tripod. Boron sits at the center, anchored firmly, and three Hydrogens spread out evenly around it in a flat plane. This arrangement isn’t random—it’s a classic trigonal planar geometry. This particular shape is what qualifies BH3 to belong to the D3h point group. The “D” refers to dihedral symmetry, “3” indicates the threefold axis, and “h” signals a horizontal mirror plane. In less jargon-heavy terms, BH3 has a very orderly, symmetrical layout.
This symmetry isn’t just fancy decoration—it determines physical and chemical properties like vibrational modes, spectral lines, and reactivity. For scientists and chemists, having a molecule in the D3h group is like knowing the secret handshake in the molecular world.
The C3 Axis: The Symmetry Spine
At the heart of this symmetry is the C3 rotation axis. It runs straight through the Boron atom—like a drill bit poking through the middle of a flat round table made of hydrogens. This axis is orthogonal, or perpendicular, to the plane hosting the hydrogen atoms. Visualize spinning the BH3 molecule around this axis by 120 degrees. The molecule looks exactly the same to anyone watching. Why? Because the three identical hydrogens swap places in a way that keeps the scene symmetrical.
But here’s where it gets spicier: there are two unique rotations related to this axis that change the hydrogen positions differently.
C3 vs. C32: Two Spins, One Axis
You might expect one rotating move per axis, but nope! The C3 axis offers two distinct symmetry operations:
- C3: A 120° turn clockwise.
- C32 (also called C3 prime): A 240° turn clockwise, or imagine rotating 120° twice.
These are not the same trick played twice—they shuffle the hydrogens into unique spots distinct from each other and the original configuration. The result? 2C3 operations are officially counted in the symmetry group, even though only one geometric axis exists. Turns out, you get double the fun with a single axis depending on which way or how much you spin.
Why Counting Symmetry Operations Matters
It might seem nitpicky to count C3 and C32 as separate, but it’s crucial. These operations influence how molecules interact with light and other chemicals, affecting their spectroscopic signatures and reactivities. So, chemists want to be precise to predict and understand experimental results. It’s like recognizing not just one dance move but every unique step in a choreography.
Seeing is Believing: Visualizing D3h Symmetry
Don’t just take my word for it. The best way to grasp D3h symmetry is to see it in action. Build a simple physical model with a Boron atom centerpiece and three Hydrogens arranged like a tripod. Spin it around the C3 axis and watch the hydrogens swap places, maintaining the molecule’s perfect symmetry.
Or for a high-tech experience, visit Symotter’s online gallery, where interactive molecular models showcase how symmetry operations work in real molecules like BH3. Watching these rotations confirms the presence of the C3 and C32 symmetry operations visually and intuitively.
Your Digital Symmetry Toolkit: Space Group Visualizer
Want to dive deeper? The “space group visualizer” is a fantastic software tool for exploring symmetry in molecules and crystals. It lets users inspect rotation axes, mirror planes, glide planes, and more across numerous point and space groups.
This is invaluable for students, researchers, or anyone curious about the symmetry universe. You can toggle operations, see atomic positions shift, and genuinely feel the rhythm of molecular symmetry in D3h and beyond. It’s like having a molecular DJ laid out on your screen.
One Axis, Two Directions: What’s Up With That?
Here’s an interesting tidbit: geometrically, D3h molecules have a single threefold rotation axis, but the symmetry group counts two different operations on this axis—rotation clockwise or counterclockwise. These are considered distinct because they produce different outcomes in atomic positioning.
Imagine a clock’s minute hand: turning it clockwise from 12 to 4 and counterclockwise from 12 to 8 doesn’t lead to the same number. Likewise, the molecule’s hydrogens land in different spots depending on rotation direction. This subtlety enriches the overall symmetry landscape of D3h molecules.
Putting It All Together: Why Care About D3h Symmetry?
Understanding the D3h point group boils down to grasping how molecules arrange and move in three-dimensional space. BH3 is a prime example, and studying its C3 axis rotations uncovers the dual nature of rotational symmetry operations.
From predicting reaction mechanisms to interpreting spectra and designing new materials, symmetry considerations like those in the D3h group help chemists and physicists unlock hidden rules of molecular behavior. They also deepen our appreciation for nature’s meticulous design.
Next time you look at a molecule, think: does it have a C3 axis? How do its atoms like to dance? That’s the power of symmetry, showing us nature’s geometric secrets one rotation at a time.
What defines the principal C3 axis in a D3h molecule like BH3?
The principal C3 axis runs through the central Boron atom. It is perpendicular to the plane that holds the three Hydrogen atoms. This axis is where the 120° rotations occur in the symmetry operations.
How many unique C3 symmetry operations exist in the D3h point group?
There are two distinct C3 operations. One is a 120° rotation (C3), and the other is a 240° rotation (C3²). Both move the hydrogen atoms into unique positions not identical to each other or the original.
Why are there two C3 operations but only one C3 axis?
Only one geometric C3 axis exists. However, the rotation can be done clockwise or counterclockwise. Each direction counts as a separate symmetry operation, so there are two distinct C3 operations.
Can real-life models confirm the D3h symmetry of BH3?
Yes, building physical models of BH3 can demonstrate its trigonal planar shape and symmetry. Online galleries and tools also allow visualization of these symmetry elements in real molecules.
What tools help visualize symmetry operations in point groups like D3h?
The “space group visualizer” software shows rotation axes, mirror planes, and glide planes in many symmetry groups. It helps users explore and understand D3h and similar groups.



Leave a Comment