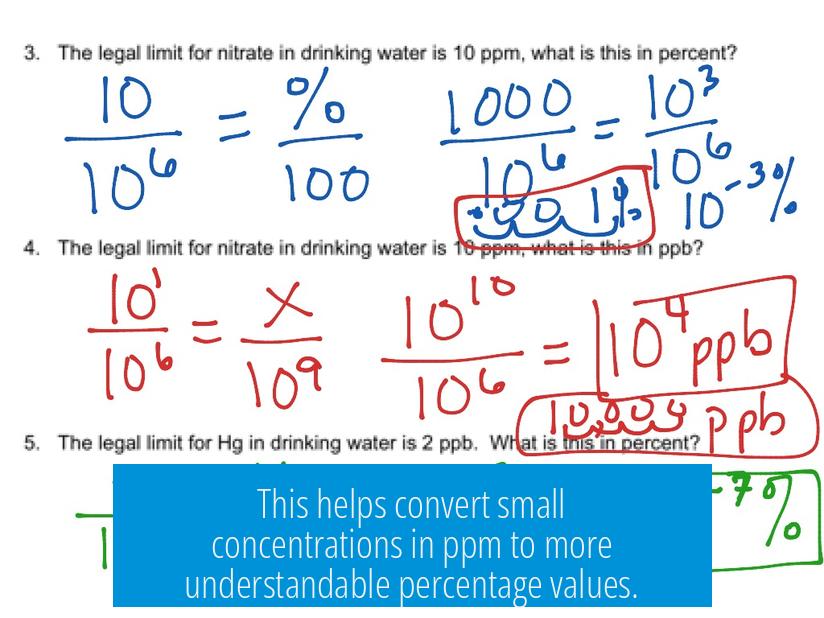
PPM to Percentage Conversion: Clear Explanation

Parts per million (ppm) converts to percentage by dividing ppm by 10,000. This means 1 ppm equals 0.0001%. The direct formula is:
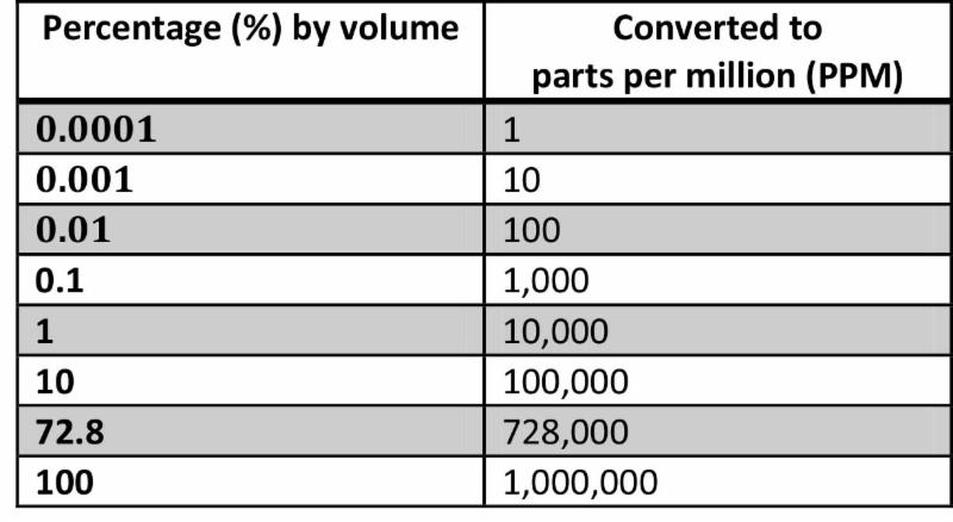
Percentage (%) = ppm ÷ 10,000
Understanding PPM and Percentage

Percentage represents parts per hundred, while ppm represents parts per million. Since 1 million is 10,000 times 100, the conversion factor is 10,000. Thus:
- 1% = 10,000 ppm
- 1 ppm = 0.0001%
This helps convert small concentrations in ppm to more understandable percentage values.
Example Calculation
Convert 60 ppm to percentage:

60 ppm ÷ 10,000 = 0.006%
This result means 60 ppm is 0.006 percent by volume or mass, depending on the context.
Common Mistakes and Notes on Units

Sometimes instructions state ppm per 10 mL, which can confuse the conversion because ppm should represent a ratio independent of sample volume.
- PPM is a unitless ratio and does not depend on the volume measured.
- Saying “ppm per 10 mL” is conceptually incorrect since ppm already specifies concentration.
- Proper interpretation requires knowing what the ppm measures (mass/volume or mass/mass).
Always check test kit instructions carefully and confirm what their ppm values reference to avoid misinterpretation.
Summary
- PPM to percentage formula: % = ppm ÷ 10,000
- Example: 60 ppm = 0.006%
- PPM is a ratio, not volume-dependent
- Verify test kit instructions for clarity
PPM to Percentage Conversion: What’s the Real Deal?
PPM to percentage conversion is straightforward: 1 ppm equals 0.0001%. Yes, it’s that simple if you know the magic behind the scenes. But don’t let that tiny number fool you. Understanding this conversion sheds light on measurements used in science, industry, and even your home water test kit.
Let’s peel back the layers and make sense of ppm and percentage, so next time someone throws “60 ppm” at you, you’ll confidently say, “Ah, that’s 0.006%!” without blinking.
What Are PPM and Percentages Anyway?
First off, percentages (% for short) mean “parts per hundred.” When you say 50%, you really mean 50 parts out of 100. It’s a simple fraction-based expression.
On the flip side, ppm stands for “parts per million.” Instead of 100 parts, ppm counts within 1,000,000 parts. Think of it as having a giant cake sliced into a million tiny crumbs. Saying 1 ppm means just one crumb.
Why does this matter? Some substances you measure come in very tiny amounts. Percentages, being parts per hundred, aren’t precise enough. For example, in water analysis or pollution data, ppm provides the detailed scale needed to spot tiny traces.
The Math: Converting PPM to Percentage
Here’s the key takeaway: to convert ppm to percentage, divide by 10,000. Or flip it – multiply percentage by 10,000 to get ppm.
- 1% = 10,000 ppm
- 1 ppm = 0.0001%
This factor of 10,000 exists because one million (ppm’s base) divided by one hundred (%) equals 10,000. It’s a simple ratio.
For example, if you’re told a water sample has 60 ppm of a certain metal, converting this to percentage is:
| Given | 60 ppm |
|---|---|
| Conversion | 60 ÷ 10,000 = 0.006% |
This means only 0.006 parts out of 100. Tiny indeed, but exactly what ppm represents.
A Real-Life Scenario: Water Test Kits and Measuring Units
Now, let’s talk practical use. A friend once got confused between ppm and volume measurements on a water test kit. Instructions said “ppm per 10 mL” — which sparked all sorts of eyebrow raises.
Here’s the problem: ppm is a ratio (parts per million parts). It’s dimensionless — no liters, no milliliters attached. Saying “ppm per 10 mL” doesn’t make scientific sense because that would be like saying “10 moles per liter per liter.” It’s redundant and confusing.
Imagine you get a measurement: 60 ppm in 10 mL of water. Does this mean 60 parts in one million parts of 10 mL? Or 60 parts per million parts in the total volume? The phrase lacks clarity.
This confusion highlights why reading instructions carefully and knowing units matter. Sometimes manufacturers write poorly constructed instructions, which only adds to the mess.
So, How Do You Handle This Confusion?
If your test kit says “ppm per 10 mL,” consider these steps:
- Check the full instructions: Sometimes the meaning emerges from broader context.
- Reach out for clarifications: Don’t hesitate to contact the supplier or manufacturer.
- Understand what exactly is being measured: Is it concentration in the sample volume? Or a separate standard?
- Use proper formulas: Convert ppm to percentage without involving volume unless volume is explicitly part of a calculation.
Sticking to dimensionless values prevents mixing units accidentally.
Why Should You Care About PPM to Percentage Conversion?
This conversion is more than just a math exercise. It’s crucial in chemistry, environmental science, and manufacturing. Knowing how tiny quantities impact products or safety standards can matter.
For example, regulatory bodies often specify safe limits of pollutants in ppm. You might wonder, “What is this in percentage terms?” Converting ppm to % helps gain perspective.
When measuring nutrients in agriculture, ppm tells the exact trace elements present. Translating that to percentage can help farmers adjust soil treatments accurately.
A Quick Recap and Handy Tip
Here’s the crème de la crème of the ppm to percentage puzzle:
- Remember: 10,000 ppm = 1%
- Formula: Percentage = ppm ÷ 10,000
- Watch units closely: PPM is unitless, don’t mix it up with volume or mass units.
- Clear confusion: Always question ambiguous instructions.
Imagine this: You want to say your water contains 45 ppm chlorine. You can also say it contains 0.0045%. It gives you flexibility and clarity when communicating chemical levels.
Wrapping It Up
Next time you stumble on ppm figures, you’ll know exactly how to translate them into percentages, avoiding those awkward moments scratching your head. And if your test kit instructions mention ppm per volume unit in a weird way, you’ll recognize that’s a red flag and proceed with caution.
Conversion is simple, but context matters. Keep your units straight, use the 10,000 factor, and you’ll master ppm to percentage conversions like a pro. No magic, just math—yet surprisingly empowering in the realms of science and daily life alike.
So, are you ready to decode ppm your next time you see it? Or has a confusing label sent you on a wild goose chase before? Let us know how you tackle these tiny but mighty numbers!



Leave a Comment