Why Round Atomic Mass to the Nearest Whole Number When Solving for Neutrons?
Rounding the atomic mass to the nearest whole number helps approximate the number of neutrons based on the most common isotope of an element. This approach simplifies calculations and aligns with the practical use of atomic mass in basic chemistry.
Atomic Mass as a Weighted Average
The atomic mass listed on the periodic table is not the mass of a single isotope. Instead, it represents a weighted average of all naturally occurring isotopes of that element. This averaging includes decimal points due to varying abundances and masses of isotopes.
Isotopes Vary in Neutron Number
Isotopes share the same number of protons but have different neutron counts. These variations cause the atomic mass to have fractional values rather than whole numbers. The atomic mass decimals reflect this mixture of isotopes and their abundance.
Rounding Targets the Most Common Isotope
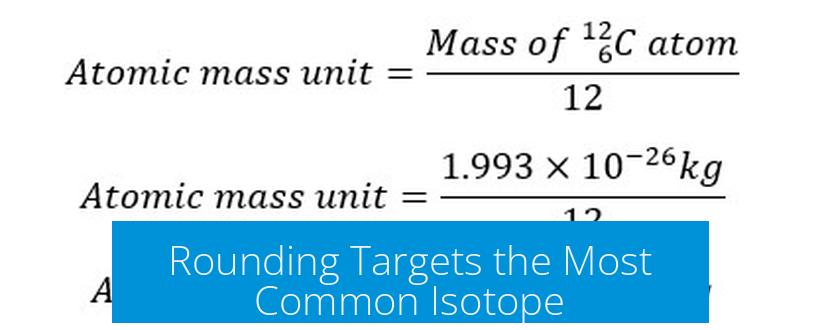
By rounding the atomic mass to the nearest whole number, one approximates the mass number of the most abundant isotope. This number equals the sum of protons and neutrons in that isotope. The neutron count then is found by subtracting the atomic number (proton count) from this rounded value.
Simplification for Learning Purposes
At an introductory or high school chemistry level, calculating neutron numbers for all isotopes is unnecessary. Focusing on the most common isotope helps students grasp the nucleus structure without added complexity. This method provides clarity and reinforces fundamental concepts.
Conceptual Understanding of the Nucleus
This rounding practice fosters awareness of the nucleus’s composition—protons and neutrons—rather than isotope diversity. It allows learners to link atomic mass, atomic number, and neutron count in a straightforward way.
Summary of Key Points
- Atomic mass is a weighted average of all isotopes; decimals result from isotope variation.
- Isotopes differ in neutron number but share the same proton count.
- Rounding atomic mass gives the approximate mass number of the most common isotope.
- Subtracting atomic number from the rounded mass yields the neutron count.
- This method simplifies learning and emphasizes nucleus structure without isotope complexity.




Leave a Comment