What Exactly Is the Difference Between Lewis Structure and Kekule Structure?
The key difference between Lewis structures and Kekule structures lies in their historical context and representation focus. Lewis structures display valence electrons and bonds using dots or lines for various molecules. Kekule structures specifically represent benzene as alternating single and double bonds with lines, emphasizing bond patterns rather than electron pairs.
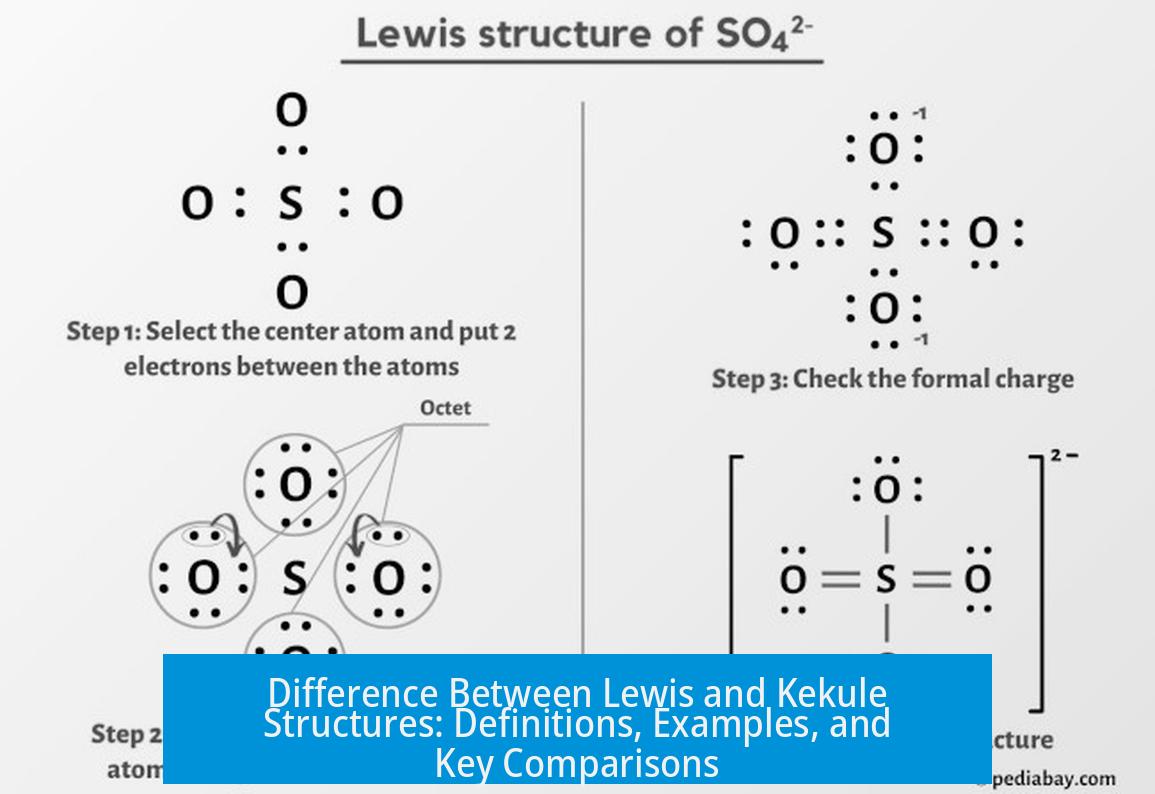
Lewis Structure: Definition and Basics
Lewis structures visually depict atoms, valence electrons, bonds, and lone pairs in molecules. Each bond appears either as a pair of dots or a line connecting atoms. Lone pairs of electrons also show as pairs of dots around atoms.
This representation helps understand electron distribution, bonding arrangements, and molecule geometry in a broad range of compounds. It suits both simple molecules and complex organic structures. Lewis structures are foundational tools in chemistry education for explaining molecular bonding.
Kekule Structure: Specific Use and History
The Kekule structure refers explicitly to the representation of benzene’s bonding proposed by August Kekule in the 1850s. He suggested that benzene consists of six carbon atoms forming a ring with alternating single and double bonds.
Rather than using dots, the Kekule structure uses lines to indicate these alternating bonds. This representation captures the pattern Kekule envisioned, inspired by a dream of a snake biting its own tail (ouroboros), symbolizing the benzene ring.
Comparison of Representation Styles
| Feature | Lewis Structure | Kekule Structure |
|---|---|---|
| Representation of bonds | Pairs of dots or lines | Lines only (single and double alternating bonds) |
| Depiction of lone pairs | Shown as dots around atoms | Typically not shown explicitly |
| Typical molecule scope | Various molecules and ions | Primarily benzene and aromatic rings |
| Historical context | Generalized bonding depiction | Specific to Kekule’s benzene model |
Resonance and Electron Delocalization
The Kekule structure presents benzene with fixed alternating single and double bonds. However, benzene is better described by resonance structures. These display delocalized pi-electrons, often symbolized by a dashed circle inside the hexagon.
Lewis structures can illustrate resonance by drawing multiple contributing forms to indicate electron delocalization. Kekule’s model predates the full understanding of resonance, focusing on localized bonds.
Overlap and Practical Use
In many chemistry contexts, the terms Lewis structure and Kekule structure overlap or are used interchangeably, especially when teaching simple organic molecules. The main distinction is that Kekule structures target benzene’s unique bonding pattern, whereas Lewis structures have a broader use.
Computational chemists and educators may refer to Kekule structures to differentiate localized bonding scenarios but often treat them as special cases of Lewis structures.
Summary of Key Differences
- Lewis structures show valence electrons, lone pairs, and bonds as dots or lines; Kekule structures depict only the alternating single and double bonds in benzene as lines.
- Kekule structures are a historical proposal for benzene’s bonding, inspired by visual motifs and predating resonance theory.
- Lewis structures apply widely across molecules; Kekule structures are almost exclusively used for aromatic compounds like benzene.
- Resonance and electron delocalization concepts extend beyond Kekule’s fixed bonding model into modern chemistry.
- Practically, Kekule structures can be seen as a subset or special case within the general framework of Lewis structures.
What is the main difference between a Lewis structure and a Kekule structure?
Lewis structures show atoms, covalent bonds, and lone electron pairs using dots or lines. Kekule structures specifically represent alternating single and double bonds in benzene using lines. The main difference is in their focus and representation style.
Why do some definitions of Kekule structures differ or seem inconsistent?
Kekule structures refer mainly to benzene’s bonding pattern with alternating bonds. Sometimes people confuse it with general Lewis structures or resonance forms. The inconsistency arises from overlap in teaching and terminology usage.
Can Kekule structures be considered a type of Lewis structure?
Yes. Kekule structures are specialized Lewis structures depicting benzene’s bonding pattern. Both represent bonding, but Kekule focuses on benzene’s alternating single and double bonds historically proposed by Kekule.
How does the Kekule structure relate to resonance in benzene?
Kekule structure shows fixed alternating bonds. Resonance structures introduce delocalized π-electrons, often depicted as a dashed circle in the ring, explaining the equal bond lengths in benzene better.
Are Lewis and Kekule structures used differently in chemistry education?
Lewis structures are widely taught for many molecules. Kekule structures mainly appear in organic chemistry when discussing benzene. Some educators may not distinguish strongly between the two terms.




Leave a Comment