Distillation of Ethanol
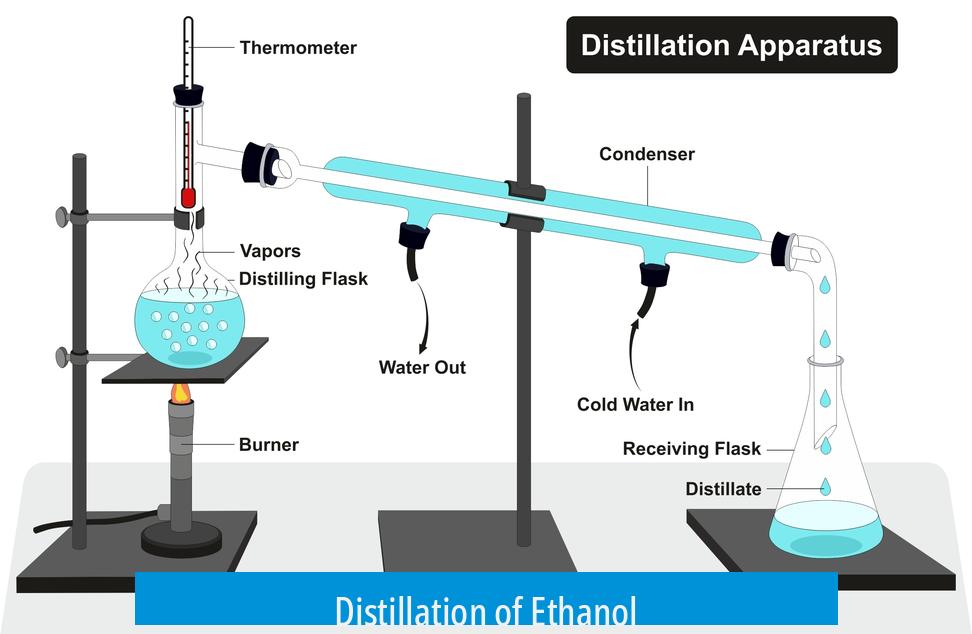
Distillation of ethanol separates it from water and other impurities based on differences in volatility and vapor pressure. While ethanol boils at approximately 78°C, water boils at 100°C, making ethanol more volatile. However, the separation process is complicated by the formation of azeotropes and the behavior of mixtures under Vapor-Liquid Equilibrium (VLE) conditions.
Understanding Volatility and Boiling Point
Volatility refers to the relative vapor pressure of a substance at a given ambient temperature. Boiling point is the temperature at which a liquid’s vapor pressure matches the surrounding pressure, allowing it to transition to the gas phase.
- Volatility predicts how easily a substance vaporizes.
- Boiling point is a measurable temperature, serving as an approximation of volatility.
- A substance with a lower boiling point generally has higher volatility, but exceptions exist.
In pure form, ethanol has a lower boiling point (~78°C) than water (100°C). Ethanol’s higher volatility means it vaporizes more readily than water under atmospheric pressure.
Behavior of Ethanol-Water Mixtures and Azeotropes
Ethanol and water form a binary mixture with unique properties. They exhibit non-ideal interactions that result in an azeotrope. An azeotrope is a mixture that vaporizes at a constant composition, where both components co-distill without separation.
- The ethanol-water azeotrope contains about 95.6% ethanol by weight.
- At this composition, the boiling point is around 78.2°C—close to pure ethanol’s boiling point.
- Because of the azeotrope, simple distillation cannot fully purify ethanol beyond ~95.6% concentration.
This azeotropic behavior limits ethanol purity from traditional distillation alone and requires additional methods like pressure-swing distillation or molecular sieves for absolute alcohol.
Vapor-Liquid Equilibrium and Raoult’s Law
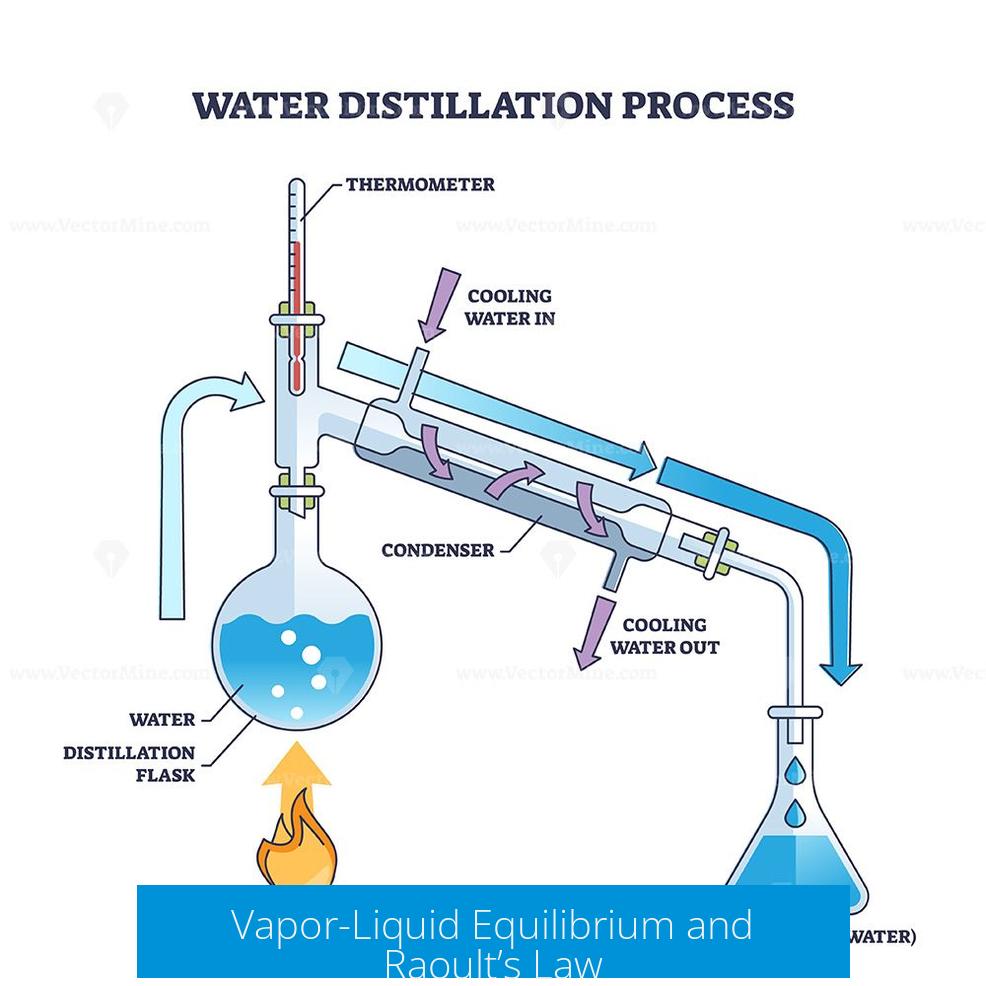
Distillation relies on Vapor-Liquid Equilibrium (VLE), describing how components distribute between vapor and liquid phases at equilibrium. Raoult’s law assumes ideal solutions where each component’s vapor pressure scales linearly with its mole fraction.
| Aspect | Description |
|---|---|
| Raoult’s Law | P_i = x_i * P_i° (Component vapor pressure in mixture = mole fraction x vapor pressure of pure substance) |
| Ideal Solution | Components obey Raoult’s law fully, no interactions beyond pure substances. |
| Real Solution | Interactions cause deviations; ethanol-water system notably deviates. |
In ethanol-water mixtures, strong molecular interactions violate Raoult’s law. Experimental VLE data guide the distillation design rather than theoretical calculations alone.
Volatility of ethanol in a mixture differs from that of pure ethanol due to these interactions, making boiling points alone insufficient for predicting separation efficiency.
Fractional Distillation and the McCabe-Thiele Method
Practical ethanol distillation uses fractional columns optimized to separate ethanol from water through multiple vapor-liquid equilibrium stages called “plates.”
- The McCabe-Thiele method is standard for designing fractional distillation columns.
- It plots equilibrium and operating lines showing vapor-liquid compositions throughout the column.
- Stepwise graphical iterations estimate the number of plates needed to achieve desired purity.
- The feed position and reflux ratio are selected to optimize separation and energy use.
This stepwise equilibrium approach models the gradual enrichment of ethanol vapor over water as vapor rises and liquid descends the column, exploiting relative volatilities at each stage.
Ethanol-Water Mixing Effects and Practical Observations
Mixing ethanol and water results in molecular interactions that cause volume contraction and heat release.
- For example, mixing 50 mL ethanol with 50 mL water yields only about 92 mL solution, not 100 mL.
- The mixture temperature slightly increases due to exothermic mixing.
- Volume change arises because ethanol and water molecules fit closely, reducing total volume.
- This effect shows mixture composition differs from simple volume ratios, affecting concentration control.
These physical changes highlight the complexities in handling ethanol-water systems beyond pure substance properties.
Common Misconceptions About Volatility and Boiling Point
Volatility is often misunderstood as the likelihood of a substance to change state. Volatility more accurately links to vapor pressure and informs boiling behavior under equilibrium conditions.
Boiling points do not fully describe the ease of separation by distillation. Interaction effects, azeotropes, and mixture composition fundamentally influence vapor-liquid balance.
Ethanol’s lower boiling point compared to water generally means it is more volatile, but during distillation its effective volatility depends on solution behavior and system pressure.
Key Takeaways
- Ethanol boils at ~78°C and is more volatile than water (100°C boiling point).
- Ethanol-water mixtures form azeotropes, preventing complete separation by simple distillation.
- Raoult’s law assumes ideal solutions; ethanol-water mixtures violate this, requiring experimental VLE data.
- McCabe-Thiele method models fractional distillation by plotting vapor-liquid equilibrium and operating lines.
- Mixing ethanol and water causes volume contraction and temperature change, showing molecular interactions.
- Boiling points alone cannot quantify distillation efficiency or substance volatility in mixtures.
What causes ethanol and water to form an azeotrope during distillation?
Ethanol and water form an azeotrope because they co-distill at a constant composition. This means their vapor and liquid phases evaporate together in a fixed ratio, making separation by simple boiling challenging.
Why is ethanol considered more volatile than water despite both being liquids?
Volatility is about vapor pressure at a given temperature. Ethanol has a higher vapor pressure and lower boiling point (~78°C) than water, so it vaporizes more readily under standard pressure.
How does Raoult’s law relate to ethanol-water distillation?
Raoult’s law predicts how vapor pressures behave in mixtures. However, real ethanol-water mixtures deviate from it, so actual separations depend on experimental vapor-liquid equilibrium data.
What practical method helps design ethanol distillation columns effectively?
The McCabe-Thiele method models vapor-liquid equilibrium on each plate within a distillation column. It estimates the number of plates and the best feed point for efficient ethanol separation.
Why does mixing equal volumes of ethanol and water result in less than their combined volume?
Molecules interact and fit tighter together, causing volume contraction. Mixing 50 mL ethanol with 50 mL water yields about 92 mL of solution, not 100 mL, with a slight temperature increase.





Leave a Comment