Removing Carbon Dioxide from the Atmosphere

Removing carbon dioxide (CO2) from the atmosphere is a complex challenge due to the vast quantity of CO2 present, the chemical stability of the molecule, and the energy required for capture. Although multiple strategies exist—including natural, chemical, and artificial methods—significant obstacles remain, making emission reduction the most crucial approach. Various methods are under research, but none provide a simple, large-scale solution yet.
1. The Scale of Atmospheric CO2 and Material Demands
The Earth’s atmosphere holds about 400 parts per million (ppm) of CO2.
Quantitatively, the atmospheric CO2 amounts to approximately 8.5 × 1015 moles from sea level up to 1 km altitude.
- Removing even 10% corresponds to about 8.5 × 1014 moles of CO2.
- Binding this quantity chemically or physically demands an enormous volume of material. For example, binding CO2 with water in a 1:1 molar ratio would require on the order of 1013 kilograms of water.
Such massive material needs make large-scale removal daunting. Producing, distributing, and managing those materials are huge logistical challenges.
Moreover, CO2 is a stable molecule with strong bonds, and it disperses evenly as a trace gas. Extracting or breaking down CO2 demands significant energy inputs, potentially negating environmental benefits if fossil fuels power such efforts.
2. Plant-Based and Natural Sequestration Strategies
Limitations of Simple Tree Planting
Planting forests sequesters CO2 but poses limitations:
- Plants release stored carbon back into the atmosphere upon decay.
- Without storing biomass permanently—such as through burial or conversion—CO2 removal is temporary.
Bio-Energy with Carbon Capture and Storage (BECCS)

BECCS harvests biomass, uses it for energy, and captures released CO2 for long-term storage.
This approach addresses the decomposition problem by preventing carbon’s return to the atmosphere.
Iron Fertilization of Oceans
Adding iron to ocean waters stimulates algal blooms that absorb CO2.
- When algae die, they sink, transferring carbon to ocean depths for centuries.
- This biological method could increase oceanic carbon uptake.
- Risks to marine ecosystems still require study.
Efficiency and Limitations of Plants
Plants are not highly efficient CO2 absorbers compared to some artificial means.
Factors include photosynthetic efficiency, growth rates, land availability, and carbon storage durability.
Concrete and Calcium Oxide (CaO) Absorption
Concrete gradually absorbs CO2 through natural carbonation.
- Pure CaO also binds CO2 chemically.
- Producing CaO from CaCO3 releases CO2, making net absorption zero or negative.
Thus, building materials can function as slow CO2 sinks, but the net climate impact depends on their production pathway.
3. Chemical and Artificial CO2 Capture Methods
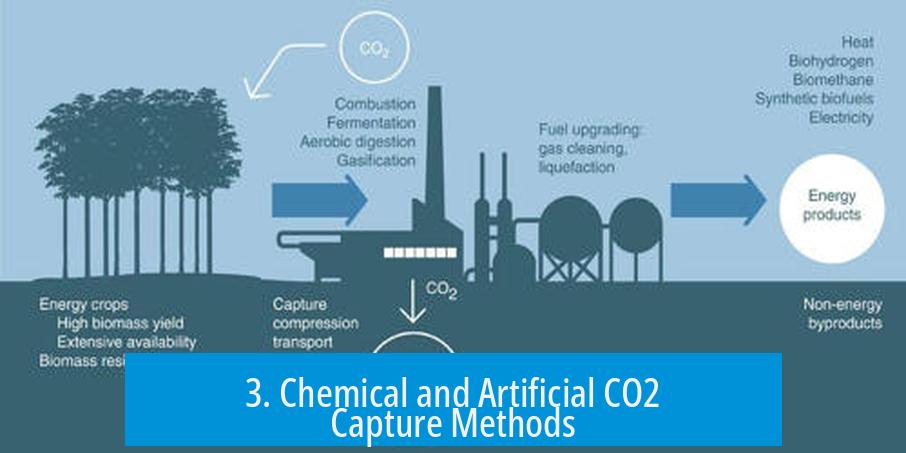
Carbon Capture as a Climate Strategy
Carbon capture is essential for meeting international climate targets, such as limiting warming to 2°C.
Industrial carbon capture focuses on intercepting CO2 emissions at their source and sometimes direct air capture.
Mechanism of CO2 Filters
- Filters use chemical agents, like amines, to bind CO2 molecules from gas streams.
- Captured CO2 can be liquified and stored underground or converted into useful chemicals (e.g., methanol, formic acid).
Challenges with Gas-Phase CO2 Binding
CO2 is chemically inert and stable, complicating direct reactions.
- Reactions typically require high temperatures or catalysts with high reactivity.
- Highly reactive materials may lack CO2 selectivity and present toxicity risks.
Electrocatalysis and the Sabatier Reaction

Electrocatalysis electrically reduces CO2 to fuels or chemicals, offering promise for low-carbon processes.
The Sabatier reaction converts CO2 with hydrogen into methane, but demands high heat and energy.
Both pathways require development to improve efficiency and scalability.
4. Risks and Limitations of Releasing Reactive Gases to Bind CO2
One speculative idea involves releasing gases that react with atmospheric CO2 to remove it directly.
- This parallels the function of filters but acts outside controlled environments.
- Released gases must be safe for breathing if dispersed widely.
- Manufacturing such gases consumes energy and may counteract benefits.
Large-scale introduction of reactive gases may disrupt atmospheric chemistry and weather unpredictably.
Such geoengineering attempts carry significant ecological and human health risks.
5. Geoengineering and Alternative CO2 Removal Approaches
Sulfur Dioxide (SO2) Injection
Injecting SO2 into the stratosphere can reflect sunlight and cool the planet.
This strategy is highly controversial due to uncertain climate side effects.
Seawater CO2 Capture
Pilot plants demonstrate extracting CO2 chemically from seawater, precipitating minerals such as magnesium carbonate.
- This mineralization locks carbon permanently.
- Direct ocean CO2 increases acidification; chemical methods avoid this.
Cryogenic Carbon Capture
With sufficient renewable electricity, CO2 can be frozen as dry ice and stored.
Energy use and container durability remain challenges.
6. Energy and Economic Considerations
Most CO2 removal approaches require large energy inputs, often from electricity or heat.
For example, producing CaO by heating limestone releases CO2, offsetting any absorption benefits.
High costs slow industrial adoption, despite environmental importance.
Industries resist expensive waste handling when no clear economic incentives exist.
7. Summary and Recommendations
- Stopping CO2 emissions is the most effective measure to reduce atmospheric levels.
- Carbon capture and storage technologies are necessary but face scientific, engineering, and economic hurdles.
- Natural methods like tree planting must include permanent carbon storage to be effective.
- Artificial approaches such as electrocatalysis and chemical filters require further research and optimization.
- Geoengineering solutions pose uncertainties and risks, needing cautious evaluation.
- Biologically inspired solutions encourage sustainable land management and biomass carbonization.
Key Takeaways
- The atmosphere holds immense CO2 quantities, making removal a huge material and energy challenge.
- Plant-based sequestration has limits; dead organic matter must be stored to keep carbon out of the air.
- Chemical capture methods revolve around binding or converting CO2 but face thermodynamic and safety constraints.
- Releasing reactive gases into the atmosphere risks toxicity and climate disruption.
- Geoengineering ideas like SO2 injection require careful risk assessment due to uncertain impacts.
- Renewable energy is critical to power CO2 removal technologies sustainably.
- Ultimately, massive emission reductions paired with strategic CO2 capture represent the balanced path forward.
Removing Carbon Dioxide From the Atmosphere: The Giant Puzzle We Must Solve
Is it possible to remove carbon dioxide (CO2) from the atmosphere on a large scale? The short answer is yes, but with huge challenges in scale, energy, cost, and unintended consequences. Let’s take a journey through the science, struggles, and hopeful strategies around this crucial task. Along the way, you’ll see why it’s *not* just about planting a few trees or spraying magic gases into the air.
Removing CO2 is one of the biggest environmental challenges ever. The amount of CO2 floating around in our atmosphere is staggering—about 400 parts per million. Though that sounds small, it translates into roughly 8.5 quadrillion moles of CO2 from sea level up to 1 km altitude. To put this in perspective: just removing 10% of that would mean dealing with 850 trillion moles of CO2. That’s a lot of molecules to catch! Imagine fitting all the CO2 in one bucket; you’d need an enormous bucket, to say the least.
The sheer scale alone spells trouble for any quick-fix idea. If we tried to bind CO2 using something common like water, a 1:1 molar ratio would require an unimaginable mass—on the order of 15 trillion kilograms of water or any other binding agent. Producing or deploying such material globally is nearly impossible, and energy demands would skyrocket.
Why Is Removing CO2 so Tricky?
CO2 molecules are stable and spread thinly across the vast atmosphere. Breaking their bonds or collecting these gas-phase molecules requires tons of energy, often more than we can spare without burning more fossil fuels—the very thing that creates more CO2!
Can Trees and Natural Methods Save the Day?
It’s tempting to think planting trees is the easy answer. But trees don’t hold carbon forever. When they die and decay, the carbon returns to the atmosphere. For plants to permanently remove CO2, the captured carbon must be stored somewhere else.
- Bio-Energy with Carbon Capture and Storage (BECCS): This is a process where biomass is grown, burned to produce energy, and the emissions captured and stored underground. It sounds tech-savvy and promising, but scaling it sustainably is a serious challenge.
- Iron Fertilization of Oceans: Sprinkling iron in the ocean triggers algae blooms that absorb CO2. When these algae die, they sink and lock carbon on the ocean floor for centuries. Cool idea, but it’s controversial due to unknown ecosystem impacts.
- Plant Efficiency: Plants aren’t the most efficient CO2 sponges. According to research, their carbon capture capacity hits natural limits. Plus, forests compete for land with agriculture and human uses.
- Concrete and CaO: Concrete and calcium oxide absorb CO2, but producing CaO releases CO2 itself. So, it’s a zero-sum game—no net carbon removal.
- Non-Biodegradable Plant Products: Using wood in durable products or cloth can lock carbon for years, but it’s limited by demand and land use.
What About Artificial and Chemical CO2 Capture?
Scientists aren’t just sitting on their hands. They’re developing filters and chemical agents that can snag CO2 right out of the air. Amines and other chemicals bind CO2, allowing it to be trapped, liquefied, or converted into useful products like methanol or formic acid.
But beware, these materials don’t react easily with CO2. The molecule is very stable. Either you need high heat to activate them or chemicals so reactive they become unsafe or unselective.
Electrocatalysis is a hot area for CO2 reduction. Here, electricity drives chemical reactions to change CO2 into fuels or other chemicals—a neat recycle, if we whip up enough renewable energy for it.
Another reaction, the Sabatier process, combines CO2 with hydrogen to create methane, but it requires serious heat and energy input.
Could We Just Release Reactive Gases to Bind CO2 in the Air?
This idea sounds tempting: let’s spray a magic gas into the atmosphere that grabs CO2. Reality check—it’s almost impossible to do safely. First, the gas must be safe for humans and animals to breathe. Second, producing the gas might cost more energy than the benefits it provides.
Even the climate effects are a big unknown. What if this gas lowers CO2 but messes with weather patterns? Are we fixing the problem or trading it for an unpredictable new disaster? This uncertainty makes releasing reactive gases a risky and generally discouraged approach.
Exploring Geoengineering and Alternative Approaches
Geoengineering ideas often sound like science fiction but are being tested seriously. For example, injecting sulfur dioxide (SO2) into the stratosphere to mimic volcanic cooling effects could reduce global temperatures. Yet, this plan is controversial because it risks unpredictable side effects and doesn’t address CO2 directly.
On the ocean front, some pilot plants are using seawater chemistry to absorb CO2. The process involves reacting CO2 with magnesium chloride in sea water to form a solid, magnesium carbonate, which can be used as eco-cement. The catch? You can’t just dump CO2 in the ocean indiscriminately, as it acidifies the water, harming marine life.
Then there’s cryogenic carbon capture, literally freezing CO2 into dry ice and storing it. This method needs huge electricity supplies, preferably renewable, to be sustainable.
Energy and Economic Barriers
Many capture methods are energy hungry. For example, to produce calcium oxide (CaO), you need to heat limestone intensely, releasing CO2 in the process. Using hydroelectric power can help, but scaling and costs remain high.
Economic hurdles are steep. Carbon capture technologies are expensive to develop and implement. Convincing companies to pick up the tab without direct profits is a major obstacle. Industries often choose the cheapest short-term option, not the planet-friendly long game.
Some methods, like concrete-based capture, end up net-zero because the CO2 released during production cancels out the absorption later.
So, What’s the Best Way Forward?
- Primary Focus on Emission Reduction: The surest, simplest message is to stop producing so much CO2 in the first place. Let the atmosphere’s natural balance process work its magic.
- Explore Multiple Strategies: Carbon capture deserves more innovative research. Artificial systems that outperform plants could hold promise, but they’re still emerging technologies.
- Learn from Nature: Chlorophyll, the green pigment in plants, has been capturing carbon for millions of years. We can harness biological cycles like tree growth, convert biomass into stable forms like biochar by pyrolysis, and bury it underground to lock away carbon long-term.
Conclusion: No Silver Bullet, But Lots of Hope
Removing CO2 from the atmosphere is a Herculean task. The scale is vast, the chemistry tough, and side effects unpredictable. There’s no magic switch. Instead, it’s a multi-front battle combining smart emission cuts, natural sequestration, emerging tech, and careful geoengineering.
Will it be easy? No. Will it be worth it? Absolutely. The planet’s future might well depend on how we innovate and integrate these approaches—thoughtfully and responsibly.
So next time you think about carbon capture, remember it’s not about one miracle idea. It’s about dozens working together, each chipping away at one of the most complex environmental puzzles of our time.





Leave a Comment