Does Iodide Have a Smell?

Iodide itself does not have a smell. Pure iodide ions in solution are odorless and colorless. However, iodide compounds can sometimes develop a faint medicinal odor over time due to the formation of trace amounts of iodine, which does have a distinctive smell.
Odor Characteristics of Iodide Solutions
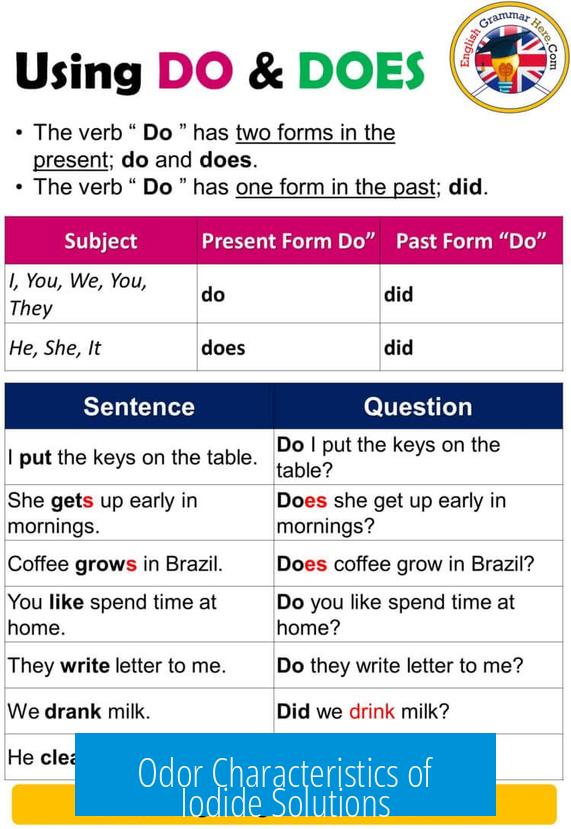
In its pure form, iodide dissolved in water appears clear and does not emit any smell. For example, potassium iodide (KI) solutions should be odorless when freshly prepared or stored briefly. The iodide ion (I−) is chemically stable and does not volatilize easily, preventing any noticeable odor.
Contrast Between Iodine and Iodide

Iodine (I2) displays a characteristic sharp odor, often compared to chlorine. This smell is familiar around pool disinfectants and cleaning agents. Bromine, another halogen element, similarly has a strong, unpleasant odor. This contrasts sharply with iodide, which lacks such an odor.
Odor in Stored Iodide Compounds
Over time, some iodide compounds like potassium iodide can develop a very mild “medicinal” smell. This odor is not from the iodide ion itself but arises because small amounts of iodine form through oxidation. Exposure to air facilitates this reaction, releasing trace iodine vapor responsible for the faint smell.
Cause of Odor Formation

- Oxidation of iodide ion produces elemental iodine.
- Elemental iodine has a distinct chlorine-like smell.
- The amount formed in typical storage conditions is low, causing only a faint odor.
- Proper storage can minimize oxidation and keep iodide odorless.
Key Takeaways
- Iodide ions are odorless and colorless in pure form.
- Iodine has a strong, chlorine-like odor; iodide does not.
- Stored iodide compounds may develop a slight medicinal odor due to iodine formation.
- Oxidation of iodide to iodine causes the odor in stored samples.
- Fresh iodide solutions remain odorless with proper storage.
Does Iodide Have a Smell? The Truth Behind the Mystery
So, does iodide have a smell? The quick, straight-to-the-point answer is: no, iodide itself does not have a smell. But as always in chemistry and life, there’s a bit more to uncover when you dig deeper into what’s really going on with this chemical compound.
Let’s dive in and sniff out the facts about iodide’s odor—or lack thereof—and clear up common mix-ups that often leave people asking, “Is that really iodide I’m smelling?” Spoiler alert: it’s probably not.
Why Iodide Is Odorless and What That Means
Pure iodide, especially when dissolved in water, is colorless and odorless. This means if you have a clear solution of iodide—say sodium iodide or potassium iodide—under normal circumstances, it should not tickle your nose or assault your senses.
For anyone working in labs or handling chemicals, this is a critical distinction. Unlike many compounds that announce their presence with a strong aroma, iodide prefers to fly under the radar. If you catch a whiff when you think you’re dealing with iodide, something else is probably happening.
The Confusing Smell of Iodine vs. Iodide
Now, let’s talk about iodine because this one’s the main culprit behind many aromatic misunderstandings. Iodine is a different beast. If you’ve ever encountered iodine, you know it has a sharp, somewhat pungent smell. Many say it smells like chlorine—a distinctive scent often linked with pool cleaning supplies.
This scent is unmistakable and quite strong, but—and here’s the kicker—it does not come from iodide. Iodide ions, which are the reduced form of iodine, are essentially smell-free.
Why does this difference matter? Iodine is a neutral element, while iodide is a negatively charged ion. They have very different chemical behaviors. The odorous smell associated with iodine vapor or solutions is simply not a characteristic of iodide compounds.
A Bromine Comparison for Context
Think of bromine, a halogen like iodine. Bromine is famous for its reddish-brown color and notorious strong odor—sharp and irritating at higher concentrations.
Similar to iodine, bromine’s elemental form smells strong and distinct. But bromide ions, like iodide ions, are odorless. It’s an interesting parallel that stresses an important chemistry principle: elemental halogens and their ionic forms can have vastly different sensory properties.
Wait, Why Does Stored Potassium Iodide Sometimes Smell Then?
If pure iodide is odorless, why does potassium iodide (KI) occasionally develop a faint medicinal smell when stored? Good question. This is where oxidation plays a sneaky role.
When KI is left exposed over time, a small fraction of iodide ions can oxidize, turning back into molecular iodine—a substance with a smell we already know well. This tiny amount of iodine can create that subtle, medicinal odor people sometimes report.
It’s not the iodide itself that smells; it’s the iodine that creeps back in during storage. This makes sense chemically and explains why fresh, pure iodide shows no odor, but older samples might.
How to Handle Iodide to Keep It Odor-Free
For people using potassium iodide or sodium iodide for medicine, supplements, or laboratory work, preventing that faint smell is often about storage conditions. Keep iodide compounds in well-sealed containers and away from air and moisture to minimize oxidation.
Fresh iodide is your best bet for an odorless experience. If your container smells even a bit medicinal, it could mean iodine is forming inside, hinting it might be time for a new batch.
Does This Smell Matter in Real Life?
Maybe you’re wondering—is the faint medicinal smell a sign of taint or hazard? Usually not. The small amount of iodine formed isn’t harmful in routine uses, but it does mean the iodide is slowly degrading. It’s like the chemical way of telling you: “Time for a refresh!”
On the flip side, some people might notice this medicinal aroma as reassuring, associating it with antiseptics and cleaning agents, which often contain iodine. This link helps explain why the iodine smell triggers strong reactions or memories.
Let’s Summarize and Clear the Air
- Pure iodide solutions are odorless and colorless—no smell, no drama.
- Iodine itself has a smell similar to chlorine—sharp and unmistakable.
- Bromine shares this trait—strong smell in its elemental form, odorless as bromide ion.
- Stored potassium iodide may develop a slight medicinal smell due to iodine forming by oxidation.
- Proper storage minimizes odors, keeping iodide fresh and smell-free.
In short, if you think you’re smelling iodide, double-check the conditions. Is it really iodide, or could a bit of iodine be playing tricks on your nose? Understanding these distinctions saves confusion and keeps your chemical experiments or health applications on point.
So next time you wonder, “Does iodide have a smell?” remember the science: it usually doesn’t, but given time and exposure, it might borrow iodine’s aromatic coat just for a while. And isn’t chemistry just full of surprises?
Does iodide have any smell in its pure form?
Pure iodide solutions are odorless. They do not emit any smell or color when freshly prepared.
Why does iodine smell but iodide does not?
Iodine has a distinct chlorine-like smell. Iodide, however, lacks this odor because it is chemically different and does not release volatile compounds.
Can potassium iodide develop a smell over time?
Yes. Stored potassium iodide may develop a faint medicinal smell. This happens because some iodide oxidizes to form small amounts of smelly iodine.
Is the smell from iodide similar to bromine?
No. Bromine releases a strong odor. Iodide itself does not smell unless it converts partially to iodine through oxidation.
What causes the faint medicinal odor in old potassium iodide?
The odor results from tiny amounts of iodine forming when iodide oxidizes over time. This iodine is responsible for the slight smell noticed.



Leave a Comment