Difference Between Electronegativity and Polarizing Power
Electronegativity refers to an atom’s general tendency to attract valence electrons, while polarizing power specifically denotes the ability of a positive ion to distort the electron cloud of a neighboring negative ion in ionic compounds.
Understanding the Concepts
Electronegativity is a broad atomic property used to describe how strongly an atom draws electrons in a bond. It applies to all atoms regardless of the bonding type. For example, fluorine has the highest electronegativity as it pulls electrons toward itself in molecules.
Polarizing power is a narrower concept that applies mainly to ionic compounds. It measures how much a positively charged ion (cation) can attract and distort the electron density of a negatively charged ion (anion). This distortion leads to partial sharing of electrons and adds covalent character to what would otherwise be purely ionic bonds.
Domain of Application
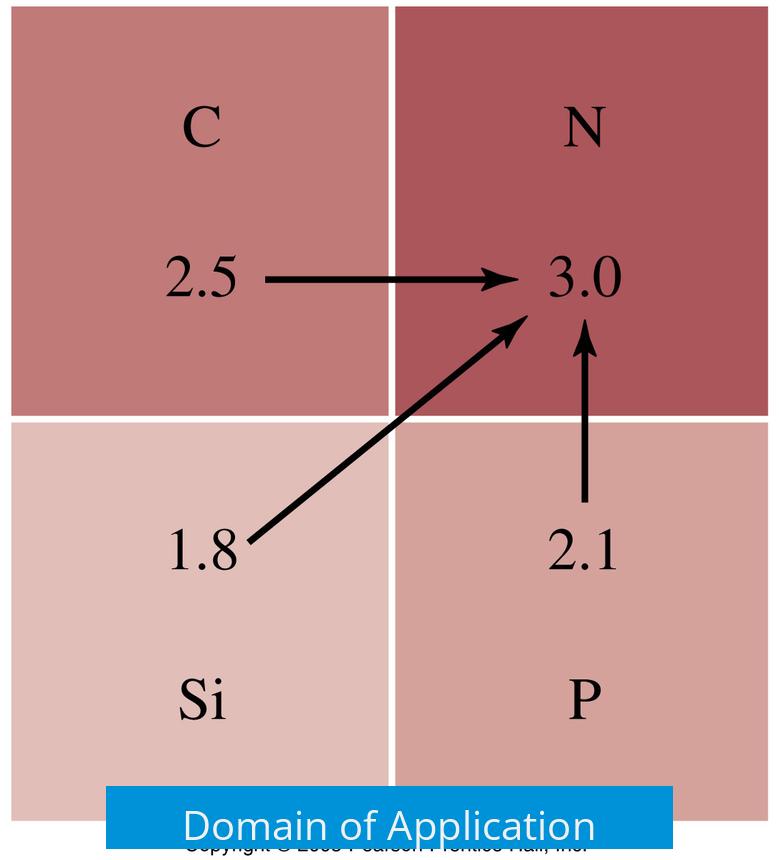
- Electronegativity: Applies to all elements and atoms, describing their general ability to attract electrons.
- Polarizing power: Relevant only for ions in ionic compounds, focusing on cations’ effect on anions’ electron clouds.
Definitions and Core Meanings
| Term | Definition |
|---|---|
| Electronegativity | Tendency of an atom to attract valence electrons toward itself in a chemical bond. |
| Polarizing Power | Tendency of a positive ion to attract and distort the electron cloud of a neighboring negative ion. |
Impact on Bonding
Electronegativity differences dictate whether chemical bonds are polar covalent or ionic. A large difference typically leads to ionic bonding, where electrons reside more with one atom. However, polarizing power affects the ionic bond’s nature by distorting electron clouds.
Even with a big electronegativity difference, the positive ion can “pull” electrons from the negative ion’s cloud, inducing covalent character within an ionic bond. This effect depends on the polarizing power of the cation and the polarizability of the anion.
Factors Influencing Electronegativity and Polarizing Power
- Electronegativity Factors:
- Number of protons in the nucleus (atomic number).
- Distance of valence electrons from the nucleus – electrons closer to the nucleus experience stronger attraction.
- Periodic trends: electronegativity increases across periods and decreases down groups.
- Polarizing Power Factors:
- Size of the cation: Smaller cations concentrate positive charge and polarize more strongly.
- Charge of the cation: Higher charges increase attraction and polarizing power.
- Polarizability of the anion: Larger, more easily distorted negative ions increase covalent character when polarized.
Summary Table of Differences
| Aspect | Electronegativity | Polarizing Power |
|---|---|---|
| Scope | General atomic property applicable to all atoms | Specific to ions in ionic compounds |
| Definition | Tendency to attract valence electrons | Tendency of a cation to attract and distort an anion’s electron cloud |
| Effect on Bonding | Determines polarity and type of bond (covalent vs ionic) | Causes distortion in ionic bonds, adding covalent character |
| Primary Influencing Factors | Atomic number, electron shell distance, periodic position | Cation size, cation charge, anion polarizability |
Application Examples
- Electronegativity: In water (H2O), oxygen’s high electronegativity pulls shared electrons, creating polarity.
- Polarizing Power: Aluminum ion (Al3+) distorts the electron cloud of oxide ions in aluminum oxide, increasing covalent character.
Why the Distinction Matters
Chemists use electronegativity to predict molecular polarity and bonding behavior broadly. Polarizing power helps explain deviations in ionic compounds from ideal ionic behavior.
This distinction clarifies why some ionic compounds show significant covalent properties. The higher the polarizing power of the cation, the more the electron cloud of the anion is shifted, reducing pure ionic character.
Key Takeaways
- Electronegativity is a general atomic property describing electron attraction in bonds.
- Polarizing power applies only to positive ions in ionic compounds affecting electron distortion.
- Electronegativity depends on protons and electron distance; polarizing power depends on ion size, charge, and anion polarizability.
- Polarizing power influences the covalent character of ionic bonds by deforming electron clouds.
- Both concepts explain different aspects of bond behavior and complement each other in chemical bonding analysis.



Leave a Comment