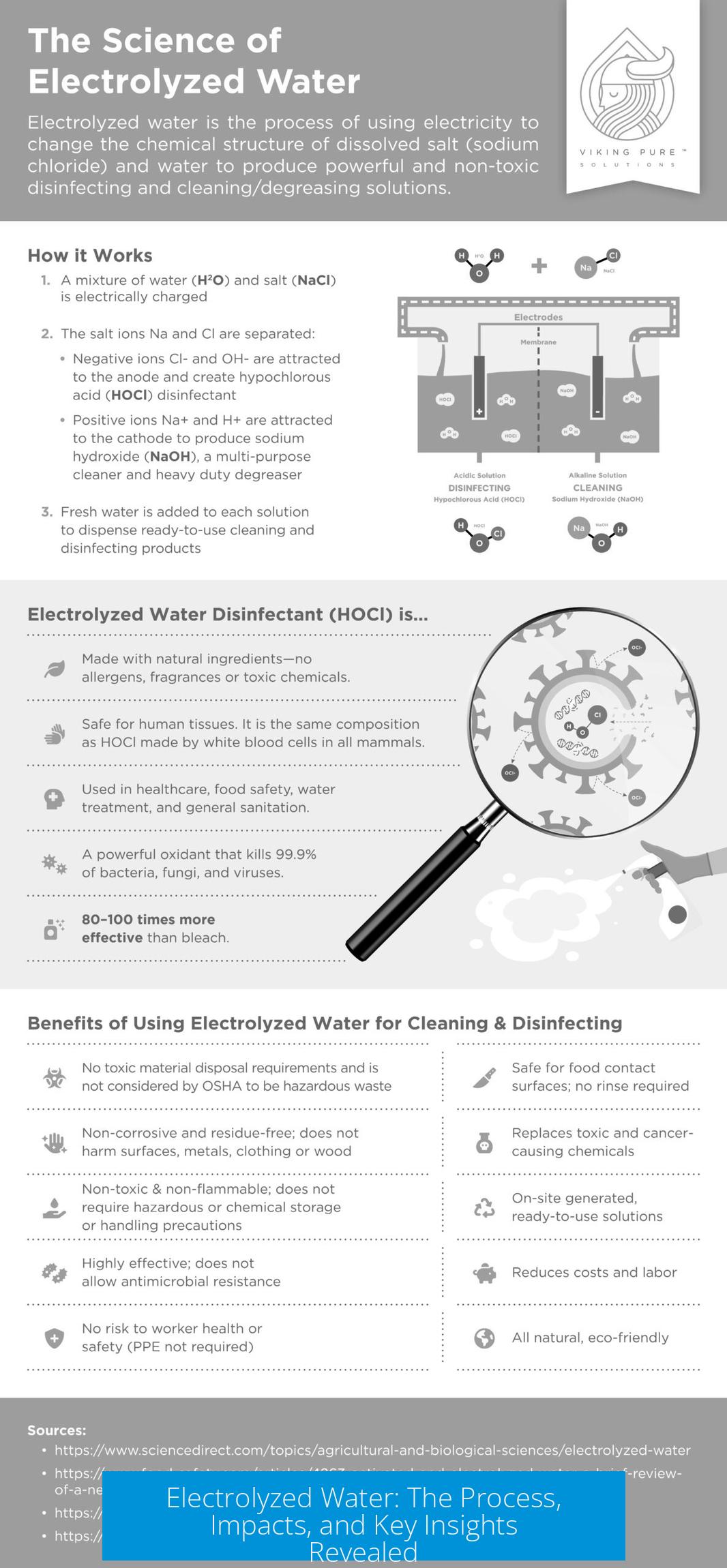
Understanding Electrolyzed Water

Electrolyzed water results from running an electric current through water containing an electrolyte, causing chemical changes mainly at the electrodes. The process alters water composition but depends heavily on the setup used.
Electrolysis Setup and Process

Electrolyzed water forms when electrical current passes through water with dissolved salt (electrolyte). The typical procedure involves:
- Using electrodes made from an iron-containing alloy rather than inert materials like platinum or MMO.
- Applying a voltage suited to drive electrochemical reactions, usually for a limited time.
- Observing the formation of residues, particularly near the anode.
Residue appears mainly due to corrosion of the anode if non-inert metal alloys are used. The amount correlates with the total current passed—higher current and longer duration increase electrode wear and residue.
Electrolyte and Electrode Material Influence

The electrolyte, commonly salt (NaCl), enables current flow via ion movement. Electrodes made of iron-containing alloys corrode under voltage, generating dark residues composed of iron oxides and similar compounds. This reaction is not typical for inert electrodes, which resist corrosion.
Thus, the nature of electrodes greatly affects the water’s final composition. Residues result mainly from electrode degradation, not the water itself.
Effect of Water Type and Mineral Content
Filtered water lacks minerals, reducing its conductivity. Lower conductivity means less current flows for the same voltage, reducing electrode corrosion and residue buildup. Hence, electrolyzing filtered water yields fewer residues.
However, filtering out minerals also strips electrolytes important for human health. Therefore, while filtered water might limit electrode corrosion, it loses beneficial minerals.
Critique and Practical Implications
This electrolysis approach risks damaging electrodes more than producing meaningful changes in water chemistry. The observed residues primarily signify electrode corrosion, not water purity or quality improvement.
Marketing claims suggesting electrolysis or specific filters create ‘better’ water may exploit such experiments. Often, this process is a sales tactic rather than a scientifically valuable water treatment method.
Key Takeaways
- Electrolyzed water forms when current passes through water containing salt electrolyte.
- Electrode material critically affects residue formation; iron alloys corrode and produce dark residues.
- Filtered water’s low mineral content lowers conductivity and electrode corrosion.
- Residue indicates electrode wear, not water improvement.
- Beware of misleading sales tactics using electrolysis to promote filters.
Electrolyzed Water: What’s Really Going On Behind the Buzz?
Electrolyzed water sounds fancy, right? It’s all the rage in cleaning, disinfecting, and maybe even drinking water circles. But what actually happens when someone says, “I ran electrolysis on water”? Is this science magic, or just a slick marketing stunt? Let’s break it down.
Imagine this: you take a glass of water, dunk in some electrodes, apply voltage, and voilà—electrolyzed water. You might think this changes the water into some super-solution that kills germs or makes your veggies healthier. But before you sign up for a $300 gadget promising miracles, here’s the scoop.
The Electrolysis Setup and Process: What Does It Look Like?
Running electrolysis on water means you stick two electrodes in a glass filled with water—often with a pinch of salt—and then zap the setup with an electric current. But not just any electrodes will do.
- Most cheap online electrolysers don’t use the high-end platinum or MMO (Mixed Metal Oxide) anodes. Instead, they rely on iron or some iron-containing alloy.
- The voltage applied and the current flow determine what happens next. If you run it longer, more intensive reactions occur. If you run it shorter or at lower voltage, less happens.
For instance, in some experiments, people see a dark residue forming, especially near the anode (positive electrode). That’s because the iron-containing anode itself slowly corrodes under the electric current. It isn’t some magic crystal forming; it’s basically electrode rot, leaving behind crud.
Why Does Residue Form? The Role of Salt and Electrode Materials
You often hear people adding salt to water before electrolysis. Salt amps up conductivity, allowing more current to flow. This increases the rate at which electrodes corrode.
In setups with salty water:
- More electric current passes through.
- Faster corrosion of iron-based electrodes.
- More residue deposits near the electrodes.
On the flip side, filtered water usually has most minerals stripped out. That means it conducts electricity poorly.
- Less current flows through filtered water.
- Less electrode corrosion.
- Less residue is observed.
But here’s the catch: this residue formation isn’t some sign of “better water quality” or a super-clean state. It’s simply electrode material dissolving into the water. Not exactly a glowing review for your “electrolyzed water” jug.
Filtered Water Vs. Mineral-Rich Water: What Really Matters?
Filtering water to strip minerals sounds good for purity, but there’s a catch. Those minerals play a crucial role in overall health. Water devoid of minerals isn’t just tasteless—it might be less beneficial.
Plus, the reduced mineral content means less electrical conductivity. That makes filtered water less reactive during electrolysis. It’s like trying to run a car on empty—things just don’t perform as expected.
So if your goal revolves around getting a magic “electrolyzed” elixir, starting with stripped mineral water might be counterproductive.
The Sales Trick: Corroded Electrodes Selling You Filters
Now here comes the eyebrow-raising part. Some marketers love showing off the corroded electrodes and dark residues to convince you about how “impure” or “bad” your water is. Then, boom—they push their filtering systems claiming to “purify” water and preserve your pipes and appliances.
Sounds clever, doesn’t it? But it’s often just a common sales trick. The corrosion isn’t a sign of your water’s villainy; it’s a reflection of the electrodes they use and the experiment’s design.
Did they just corrode the electrodes on purpose? Yup. To scare you into buying filters. The dark sludge left behind isn’t some science behind a miracle cure; it’s a carefully-crafted fear tactic to boost sales.
So What’s the Takeaway on Electrolyzed Water?
If you ask, “What is electrolyzed water?”—it’s water that’s been exposed to an electric current, causing chemical changes. But the changes depend heavily on what’s in your water and what kind of electrodes you use.
Quality electrodes, proper electrolytes, and controlled voltage produce specific forms of electrolyzed water known for disinfecting or cleaning. Cheap setups using iron alloys end up corroding, leaving behind doubtful residues, not miracle water.
Moreover, filtered water might resist corrosion but loses essential minerals in the process. So stripping away minerals to get “better” electrolysis results might defeat the purpose of drinking healthy water.
Practical Tips If You’re Curios About Electrolyzed Water:
- Use electrodes made from stable materials like platinum or MMO if you want reliable electrolysis results.
- Understand that adding salt boosts electricity flow but accelerates electrode corrosion.
- Don’t fall for scary residue photos—they usually just show electrode decay, not poisonous water.
- Remember, minerals in water are good for your health—don’t strip them indiscriminately.
- If your goal is sanitizing surfaces, electrolyzed water can be effective but depends on the exact chemistry.
- Before investing in expensive ionizers or electrolysis devices, research what kind of electrodes and electrolyte they use.
Electrolyzed water isn’t a silver bullet, but it can be useful. Just make sure you know what you’re getting. Otherwise, you could be buying fancy gadgets that corrode your electrodes and confuse your taste buds.
Ready to test your curiosity? Next time you try electrolysis, peek at your electrodes closely. Are they pristine or rusting away? That residue? It’s probably the electrode saying, “Hey, don’t abuse me like this!”
In a world buzzing with “cleansing,” “detox,” and “electro-” gimmicks, a little science helps you stay one step ahead. Because, as it turns out, sometimes water is just water—and the magic is in how you treat it, not in some techno-spell. Cheers to clear understanding and hydrated adventures!
What causes the dark residue during water electrolysis?
The dark residue comes from the anode material, which in many DIY setups is made of iron alloy. This material corrodes during electrolysis, creating the visible residue. The residue amount depends on the current and duration of electrolysis.
How does water mineral content affect electrolysis?
Water with more minerals conducts electricity better, causing more electrode corrosion. Filtered water has fewer minerals, reducing current flow and residue. However, filtering removes useful minerals from water, which is generally not beneficial.
Is electrolysis useful for water purification?
Electrolysis mainly causes electrode corrosion and residue formation. It is not an effective water purification method and is sometimes misused in marketing to promote filters by showing electrode corrosion as contamination removal.
What type of electrodes are typically used in home electrolysis setups?
Home electrolysis kits often use iron-based alloy electrodes instead of platinum or MMO. These cheaper electrodes corrode easily, producing residue during experiments, unlike professional-grade electrodes that resist corrosion.



Leave a Comment