Difference Between Ethanol and Isopropyl Alcohol (IPA): Molecular Structure and Its Effects
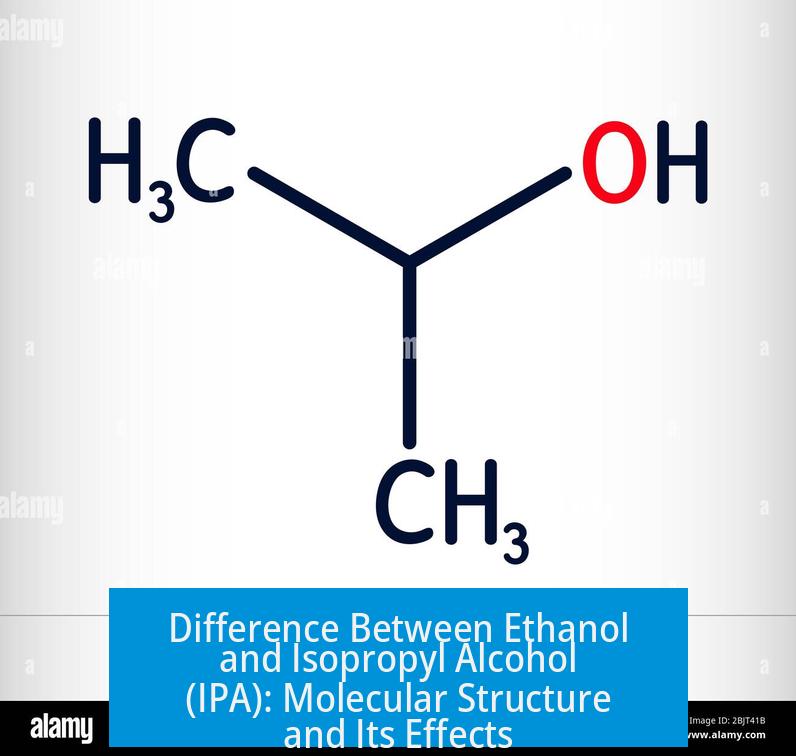
Ethanol and isopropyl alcohol differ in molecular structure by a single methyl group, which significantly impacts their chemical properties, biological effects, and applications even when both are at the same purity level.
Both are alcohols featuring a hydroxyl (-OH) group, but ethanol (EtOH) consists of two carbon atoms, while isopropyl alcohol (IPA), or isopropanol, contains three carbons due to an additional methyl group attached to the central carbon. This difference alters their polarity, toxicity, metabolism, boiling points, and solvent properties, thereby justifying their diverse uses in industry, laboratories, and everyday products.
1. Molecular Structure and Chemical Properties
Ethanol’s chemical formula is C2H5OH. IPA’s formula is C3H7OH, reflecting an extra methyl group attached to the central carbon atom where the hydroxyl group resides.
- Structural Difference: IPA has an extra -CH3 group compared to ethanol.
- Effect on Polarity: The additional methyl group makes IPA less polar than ethanol.
This reduced polarity affects properties such as solvent behavior, miscibility with water, and boiling point. IPA’s higher non-polar character means it can dissolve more hydrophobic substances compared to ethanol, which is more hydrophilic.
2. Physical Differences Due to Structure
| Property | Ethanol (EtOH) | Isopropyl Alcohol (IPA) |
|---|---|---|
| Molecular Formula | C2H5OH | C3H7OH |
| Molecular Weight (g/mol) | 46.07 | 60.10 |
| Boiling Point (°C) | 78.37 | 82.6 |
| Polarity | More polar | Less polar (more non-polar) |
| Water Miscibility | Completely miscible | Completely miscible |
Because IPA has a higher boiling point, it is used in applications requiring higher temperature tolerance and slower evaporation rates than ethanol, such as certain recrystallization procedures.
3. Biological and Metabolic Differences
When ingested, ethanol and IPA undergo different metabolic pathways, yielding distinct metabolites with varying toxicity levels.
- Ethanol: Metabolized primarily into acetaldehyde by alcohol dehydrogenase enzymes, then further to acetic acid. Acetaldehyde is toxic and contributes to hangover effects and liver damage in chronic use.
- Isopropyl Alcohol: Converted mainly to acetone, which is less reactive and more difficult to metabolize further. The exact metabolic impacts of acetone are less toxic but can accumulate and cause harmful effects.
Animal studies indicate IPA is acutely more toxic. For example, the median lethal dose (LD50) in rats is approximately 5 g/kg for IPA compared to 12 g/kg for ethanol. This implies IPA poisoning requires less quantity.
Human exposure to IPA, whether accidental or intentional, causes faster onset of central nervous system depression, respiratory irritation, and higher toxicity risks. Its ingestion is more hazardous than ethanol, which humans can tolerate in larger amounts due to adaptive metabolism.
4. Solvent Properties and Applications
The structural and polarity differences create distinct solvent profiles, influencing their use in laboratories, pharmaceuticals, and industrial processes.
- Ethanol
- Used as a universal solvent for both polar and somewhat non-polar compounds.
- Preferred in biological and food-grade applications due to lower toxicity and regulatory approval for human consumption.
- Widely used in beverages, pharmaceuticals, and DNA precipitation techniques in molecular biology, where ethanol produces a characteristic color to DNA pellets.
- Isopropyl Alcohol (IPA)
- More effective for dissolving non-polar and hydrophobic substances due to its increased non-polar character.
- Favored in cleaning disinfectants, degreasers, and transfer hydrogenation catalysis where acetone formation is preferable over acetaldehyde.
- Commonly used in clinical and industrial settings for skin antiseptics but unsuitable for ingestion.
- In chromatography, IPA can aid separation of analytes with non-polar characteristics, complementing ethanol or other solvents.
5. Cost, Purity, and Regulatory Considerations
Ethanol’s use in consumables can make it expensive and subject to strict regulation. It often contains denaturants or impurities such as methyl ethyl ketone (MEK), toluene, or methanol where purity is critical but tax avoidance is necessary. Pharmaceutical or reagent-grade ethanol is costly but necessary for sensitive chemical or biological procedures.
IPA is generally more affordable, widely available in high purity, and less regulated due to its non-consumable nature. For laboratory solvent uses that do not involve ingestion, IPA offers a practical alternative.
6. Practical Examples of Different Uses
- Medical Disinfectants: IPA is commonly used due to its effective microbial action and skin tolerability without systemic toxicity from ingestion.
- Pharmaceutical Formulations: Ethanol is used in oral medications, tinctures, and flavorings because of its safer metabolic profile and palatability.
- Laboratory Solvent Extraction: IPA’s non-polar properties suit extraction of hydrophobic compounds better than ethanol.
- Chromatography: IPA serves as a mobile phase modifier for certain HPLC separations, where analyte polarity demands it over ethanol.
- Molecular Biology: Ethanol precipitates nucleic acids with clearer outcomes than IPA, which can alter DNA pellet appearances.
Summary: Key Takeaways
- Structural Difference: IPA has an extra methyl group making it less polar and heavier than ethanol.
- Toxicity: IPA is more acutely toxic and metabolized into acetone; ethanol converts to acetaldehyde.
- Boiling Points: IPA’s higher boiling point favors its use in higher-temperature applications.
- Solvent Uses: IPA better dissolves non-polar compounds; ethanol suits polar to moderately non-polar substances.
- Biological Impact: Ethanol is safe for consumption at regulated levels; IPA is not suitable for ingestion and mainly used externally.
- Cost and Purity: Ethanol is more expensive due to regulatory and purity standards; IPA is cheaper and readily available in reagent-grade.
1. How does the molecular structure difference between ethanol and IPA affect their uses?
IPA has an extra methyl group compared to ethanol.
This makes IPA more non-polar.
The difference changes their solvent properties and biological effects.
2. Why is IPA considered more toxic than ethanol despite similar purity?
IPA is metabolized into acetone, which is harder to break down.
Ethanol turns into acetaldehyde, a toxic but more familiar metabolite.
Less IPA is needed to cause fatal toxicity compared to ethanol.
3. Does the difference in boiling point between IPA and ethanol influence their lab applications?
Yes, IPA has a higher boiling point.
This helps in reactions needing higher temperatures or specific recrystallizations.
Ethanol boils at lower temperatures, limiting its use in some heating steps.
4. How does the polarity difference impact their solvent roles?
IPA’s extra methyl group makes it more suitable for non-polar compounds.
Ethanol works better with polar substances.
This guides chemists when choosing a solvent for extraction or chromatography.
5. Are there any practical differences in biological or laboratory outcomes using IPA vs ethanol?
In molecular biology, DNA pellets differ in color with each alcohol.
Ethanol is drinkable and preferred for consumables; IPA is not.
IPA is used in specific catalytic reactions due to cleaner byproducts.




Leave a Comment