Comparison of TsOH and H2SO4
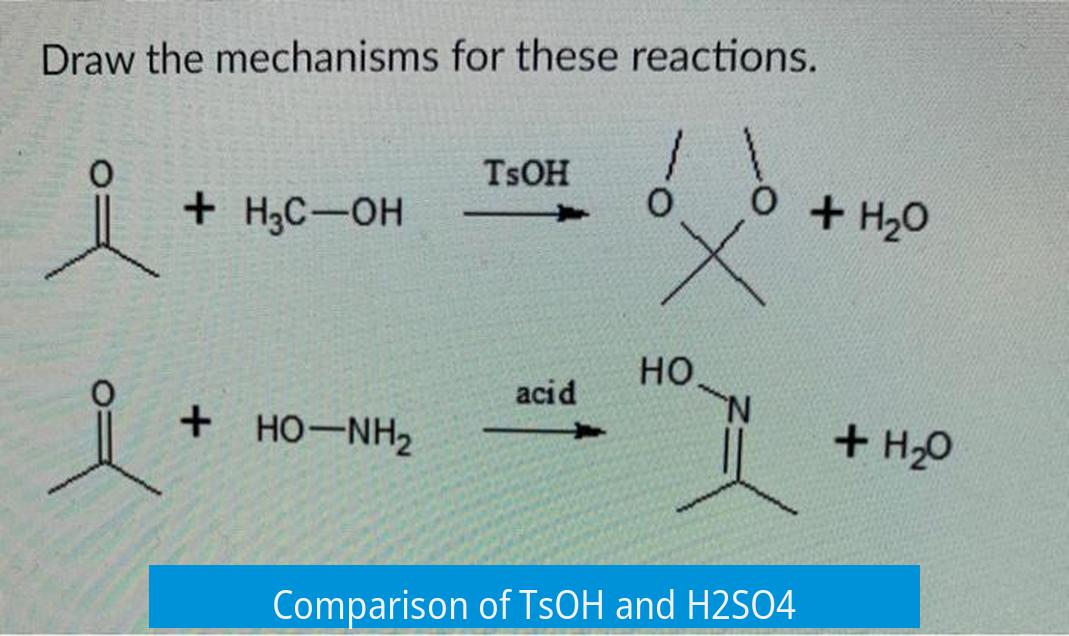
TsOH (p-Toluenesulfonic acid) is a milder acid than sulfuric acid (H2SO4), offering easier handling, superior solubility in organic solvents, and cleaner reaction outcomes, especially in organic synthesis. H2SO4 is stronger but harsher and prone to side reactions.
1. Acid Strength and Chemical Properties
TsOH is a sulfonic acid with moderately strong acidity but less than sulfuric acid. Its acid strength allows protonation without aggressively oxidizing or degrading sensitive substrates. In contrast, sulfuric acid is a highly strong acid and an oxidizing agent. This strength can provoke unwanted side reactions, such as polymerizations or decompositions, especially when acid-sensitive functional groups are present.
Because of its milder nature, TsOH favors controlled protonation with fewer byproducts. H2SO4 often leads to harsher reaction conditions and sometimes complicates product isolation due to side reactions.
2. Physical State and Handling Convenience
- TsOH: Exists as a solid at room temperature, often appearing as a white powder or crystalline solid.
- H2SO4: A dense, viscous liquid, frequently used in an aqueous form.
The solid nature of TsOH makes it easier to weigh accurately and handle safely without exposing the operator to corrosive liquid droplets or vapors. Sulfuric acid liquid form often contains water, adding complexity when precise anhydrous conditions are required. TsOH’s lower water content reduces complications from solvent effects or dilution during reaction setup.
3. Solubility in Organic Solvents and Reaction Efficiency
TsOH’s solubility profile is a significant advantage in many organic reactions. It dissolves well in common organic solvents such as benzene, toluene, and alcohols. This enhanced solubility promotes better interaction with organic substrates or catalysts, improving reaction rates and yields.
Sulfuric acid’s high polarity and ionic character cause poor solubility in many organic solvents. This limitation often requires aqueous or biphasic systems, complicating reaction control and sometimes leading to emulsions or difficult separations. Additionally, sulfuric acid can co-distill with water in azeotropic setups, complicating reaction design—TsOH largely avoids this issue and remains stable under similar conditions.
4. Application-Specific Advantages
TsOH is frequently preferred in esterification and etherification reactions, such as the Williamson ether synthesis and Fischer esterification. Its milder acidity helps avoid side reactions, giving cleaner products and easier purification steps. For example, when synthesizing ethers or esters, TsOH yields higher purity by minimizing dehydration or rearrangements common under stronger acids.
H2SO4 often induces side reactions due to its strong acidity and oxidizing properties. Functional groups sensitive to strong acids may degrade or rearrange, reducing yields or yielding complex mixtures. In such cases, TsOH enables retention of acid-sensitive functionalities by providing gentler reaction conditions.
Other acids sometimes surpass TsOH depending on the context. For example:
- Camphorsulfonic acid (CSA): Known for higher acidity and chiral properties, may outperform TsOH in some asymmetric syntheses.
- Polyphosphoric acid: Used in specialized esterifications, can offer advantages over sulfuric acid.
Nevertheless, TsOH balances acid strength, handling, and solubility, making it a versatile choice in organic synthesis.
5. Removal and Post-Reaction Handling
TsOH is easier to remove after reactions due to its solubility and lower corrosiveness. It can be washed out or neutralized efficiently without harsh conditions. In contrast, sulfuric acid’s strong acidity and water content make neutralization more challenging and may require careful control to prevent product degradation or acid contamination.
This ease of removal reduces time and cost in workup steps, benefiting synthetic workflows.
Summary Table: TsOH vs H2SO4
| Feature | TsOH (p-Toluenesulfonic Acid) | H2SO4 (Sulfuric Acid) |
|---|---|---|
| Acid Strength | Milder acid; fewer side reactions | Stronger acid; prone to side reactions and oxidation |
| Physical State | Solid; easier to weigh and handle | Liquid; often aqueous, corrosive |
| Solubility | Highly soluble in organic solvents | Poorly soluble; co-steam distillation with water |
| Application | Cleaner products in esterifications, etherifications | Can cause unwanted reactions; harsher |
| Removal | Easy to remove post-reaction | Difficult due to corrosiveness and high acidity |
Key Takeaways
- TsOH is a milder sulfonic acid, suitable for delicate organic syntheses sensitive to strong acids.
- Solid-state TsOH offers handling advantages over liquid sulfuric acid.
- TsOH’s high solubility in organic solvents enhances reaction efficiency.
- TsOH provides cleaner outcomes in esterification and etherification reactions.
- Sulfuric acid’s strong acidity enables certain reactions but risks side reactions and difficult post-reaction handling.
What makes TsOH milder than H2SO4 in chemical reactions?
TsOH is less acidic and less likely to trigger side reactions compared to H2SO4. It is gentler on acid-sensitive groups, helping maintain their integrity during reactions.
Why is TsOH preferred over H2SO4 for organic solvent reactions?
TsOH dissolves better in organic solvents, allowing more effective contact between catalyst and reactants. H2SO4, being less soluble, can limit reaction efficiency in these solvents.
How does the physical state of TsOH and H2SO4 affect their handling?
TsOH is a solid, which makes it easier to measure and handle with less water content. H2SO4 is a liquid often mixed with water, complicating its use and removal afterward.
In which reactions does TsOH provide cleaner results than H2SO4?
TsOH is favored in Williamson ether synthesis and Fischer esterifications because it reduces unwanted side reactions, leading to cleaner products.
Why is TsOH easier to remove from reaction mixtures compared to H2SO4?
Due to its solid form, milder acidity, and lower water content, TsOH can be removed more simply. H2SO4’s strong acidity and association with water make its removal tougher.



Leave a Comment