Can It Be Generalized That a Single Bond = sp3, Double Bond = sp2, and Triple Bond = sp?

The simple generalization that single bonds correspond to sp3 hybridization, double bonds to sp2, and triple bonds to sp is mostly true but not universally correct. Hybridization depends on the number of electron domains—sigma bonds plus lone pairs—around an atom rather than the bond order alone.
Understanding Hybridization Beyond Bond Type
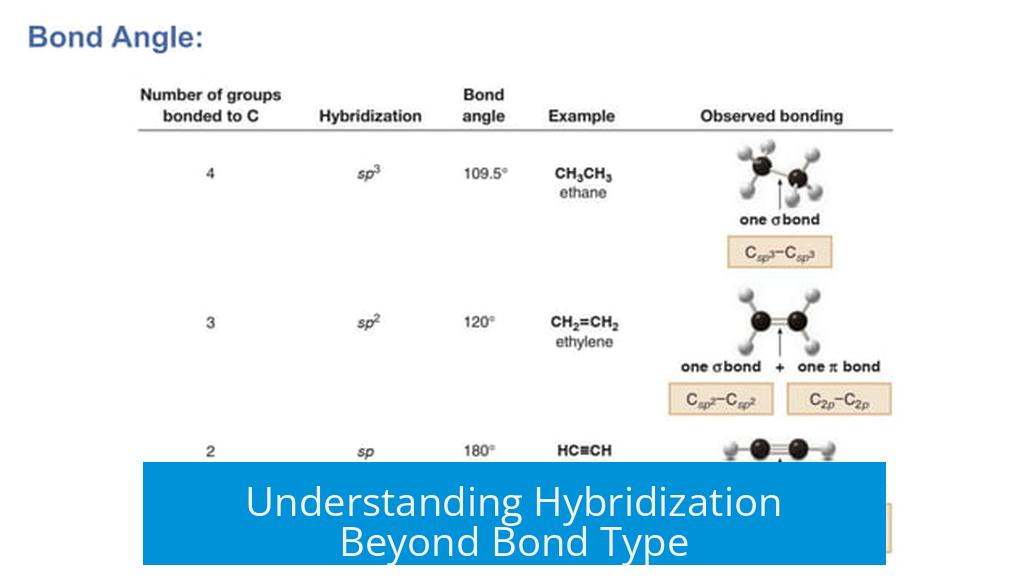
Hybridization describes the mixing of atomic orbitals to form new hybrid orbitals for bonding. These orbitals influence molecular geometry and bond properties. In organic chemistry, the common model connects bond types with hybridization:
- Single bond → sp3
- Double bond → sp2
- Triple bond → sp
This model serves as a helpful rule of thumb, especially for carbon atoms with standard bonding patterns.
Why the Generalization Can Fail
The generalization breaks down because hybridization depends on the steric number: the count of sigma bonds plus lone pairs on the atom. This means bonding patterns alone can’t fully define hybridization.
For example:
- In boron hydride BH3, boron forms three single bonds but is sp2 hybridized because it has only three electron domains and no lone pairs.
- In allene (H2C=C=CH2), the two terminal carbons are sp2, but the central carbon is sp hybridized despite multiple single and double bonds nearby.
Determining Hybridization: The Correct Approach
The accurate method counts all groups attached to the atom, including lone pairs, responsible for electron domains:
| Steric Number (Electron Domains) | Hybridization | Explanation |
|---|---|---|
| 4 | sp3 | Four sigma bonds or combinations of bonds and lone pairs (e.g., methane) |
| 3 | sp2 | Three sigma bonds/lone pairs, typically involves a double bond (e.g., ethene) |
| 2 | sp | Two sigma bonds/lone pairs, as in triple bonds or linear molecules (e.g., acetylene) |
Importantly, pi bonds do not affect hybridization count; only sigma bonds and lone pairs do.
Examples of Hybridization Variations
- Carbon with 4 single bonds: Four sigma bonds → steric number 4 → sp3 hybridization.
- Carbon with 1 double bond and 2 single bonds: Two single sigma bonds + one double bond’s sigma bond = three electron domains → sp2.
- Oxygen atom with 2 bonds and 2 lone pairs: Four electron domains → sp3 hybridized.
Exceptions and Special Cases
Some atoms and functional groups deviate from this rule due to elemental restrictions or resonance effects:
- Boron: Boron often forms three bonds with no lone pairs, resulting in sp2 hybridization even without double bonds.
- Amides Nitrogen: Despite having a steric number of 4 suggesting sp3, resonance delocalization leads the nitrogen lone pair to interact with the carbonyl system, making the nitrogen effectively sp2 hybridized.
Practical Advice for Organic Chemistry Students
For most introductory organic chemistry settings, applying the generalization is sufficient. Students should anticipate rare counterexamples but understand them as exceptions rather than the rule.
Stepwise method for hybridization assignment:
- Count all sigma bonds on the atom.
- Include all lone pairs on the atom.
- Sum these to get the steric number.
- Assign hybridization: steric number 4 = sp3, 3 = sp2, 2 = sp.
- Ignore pi bonds—they do not affect hybridization count.
This approach minimizes errors and clarifies molecular shape and reactivity.
Summary of Key Points
- Single, double, and triple bonds often relate to sp3, sp2, and sp hybridization but not always.
- Hybridization depends on the number of sigma bonds plus lone pairs (steric number), not on bond order alone.
- Pi bonds do not count towards hybridization determination.
- Exceptions include atoms like boron and resonance-influenced atoms such as amide nitrogens.
- Counting electron domains provides a reliable method for accurate hybridization assignment.
- The generalization remains a useful learning shortcut but should be refined for advanced understanding.
Can a single bond exclusively indicate sp3 hybridization?
No. Single bonds can form between atoms with sp3, sp2, or sp hybridization. Hybridization depends on electron domains, not just bond order.
Why is the rule “single bond = sp3, double bond = sp2, triple bond = sp” not always correct?
This rule ignores lone pairs and electron domains. Hybridization depends on steric number, the total of bonded atoms plus lone pairs, causing exceptions.
How do lone pairs affect hybridization assignment?
Lone pairs count as electron domains. For example, oxygen is sp3 hybridized due to two bonds and two lone pairs, even without multiple bonds.
Can atoms other than carbon follow the single bond = sp3 rule?
No. Atoms like boron, which form fewer bonds, may have different hybridizations despite single bonds, such as sp2 in BH3.
How do resonance structures influence hybridization?
Resonance can change effective hybridization. For instance, amide nitrogen may be sp3 by steric number but behaves as sp2 due to lone pair delocalization.




Leave a Comment