Will Allylic and Benzylic Halides Prefer SN1 or SN2 Reaction?
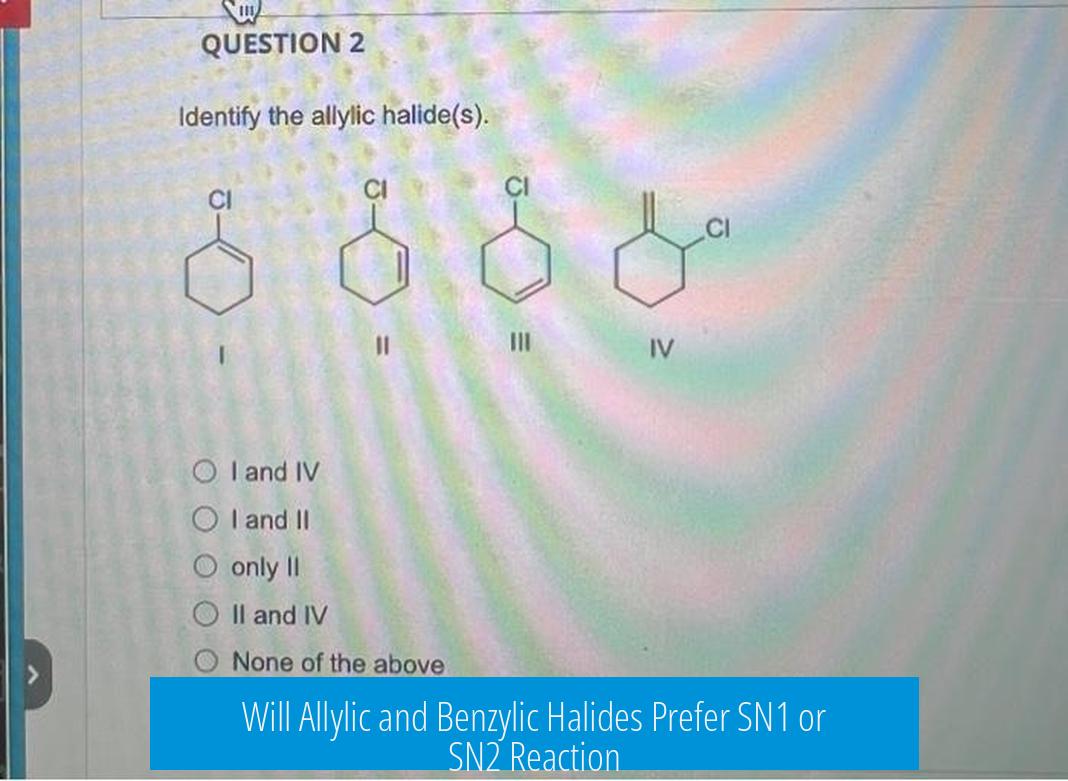
Allylic and benzylic halides can undergo both SN1 and SN2 reactions, but their preference depends on factors such as carbocation stability, nucleophile strength, solvent, and substituents. Generally, benzylic halides favor SN1 due to better carbocation stabilization, while allylic halides lean toward SN2 or SN2′ mechanisms.
Carbocation Stability and SN1 Preference
Both allylic and benzylic carbons can form resonance-stabilized carbocations. This stabilization promotes SN1 reactions under suitable conditions by lowering the activation energy of carbocation formation. Benzylic carbocations are more stabilized by the aromatic ring’s resonance, making SN1 pathways more accessible, especially in the presence of electron-donating groups on the aryl ring.
Factors Affecting Reaction Pathways
- Nucleophile strength: Strong nucleophiles favor SN2, while weak nucleophiles promote SN1.
- Solvent polarity: Polar protic solvents stabilize carbocations, supporting SN1; polar aprotic solvents favor SN2.
- Halide substitution: Primary halides tend to undergo SN2; secondary and tertiary favor SN1.
Benzylic vs. Allylic Halides
Benzylic halides more often undergo SN1 reactions because the adjacent aromatic ring stabilizes the carbocation effectively. Allylic halides, generally primary or secondary, tend to react via SN2 or SN2′ mechanisms due to less steric hindrance and accessible reaction sites.
Structural and Kinetic Considerations in SN2

SN2 reactions proceed via backside attack on an exposed carbon. The primary nature of allylic and benzylic carbons, combined with planar aromatic rings, allows nucleophiles to approach more easily. This structural accessibility often results in faster SN2 kinetics compared to SN1, which requires carbocation formation and is hindered by steric factors.
Influence of Substituents on Benzylic Halides
Substituents on the aromatic ring modulate the reaction mechanism:
- Electron withdrawing groups (EWG): Decrease carbocation stability, pushing the pathway toward SN2.
- Electron donating groups (EDG): Stabilize carbocations, favoring SN1.
Linear Free Energy Relationships (LFER) studies confirm these trends, demonstrating predictable shifts in mechanism based on substituent effects.
Key Takeaways
- Both halides can undergo SN1 and SN2; the pathway depends on conditions.
- Benzylic halides favor SN1 due to strong carbocation stabilization.
- Allylic halides usually prefer SN2 or SN2′ because of less steric hindrance.
- Strong nucleophiles and polar aprotic solvents promote SN2.
- Substituents on benzylic halides modulate the mechanism via electronic effects.
Will Allylic and Benzylic Halide Prefer SN1 or SN2 Reaction?
Allylic and benzylic halides have a foot in both camps—but the preference between SN1 and SN2 largely depends on the structure, reaction conditions, and subtle electronic effects. Let’s unpack this chemical conundrum in a way that makes sense, even if you’re not wearing your lab coat today.
Imagine you’re at a party, and the nucleophile wants to “attack” an electrophilic carbon. The question is: Do they sneak in with a quick one-step strike (SN2), or wait patiently while the carbon leaves the party temporarily, forming a carbocation intermediate (SN1)? With allylic and benzylic halides, the answer isn’t a simple yes or no—it’s a bit of a “depends.”
Carbocation Stability: The Heavyweight in SN1 Reactions
Both allylic and benzylic halides can form stabilized carbocations. What does that mean? The carbocation formed after the halide leaves finds some serious resonance support in the adjacent double bond (allylic) or aromatic ring (benzylic). This resonance stabilization is like giving the carbocation a comfy armchair to rest on, reducing its energy and making SN1 a viable path.
So, if the conditions favor carbocation formation—like a polar protic solvent that stabilizes ions, or a weak nucleophile—these halides can comfortably go SN1. But it’s not always a partying-goes-on scenario. The reaction tends to be slower if the carbocation is quite stable, which sometimes feels like kicking the can down the road before the nucleophile finally attacks.
Allylic vs. Benzylic: Who Prefers What?
Benzylic halides often lean more towards SN1 reactions. This preference is because the benzyl carbocation is exceptionally stable, thanks to the aromatic ring’s resonance. The aromatic ring doesn’t just offer a placebo effect; it truly stabilizes the positive charge, making carbocation formation easier and more favorable.
In contrast, allylic halides often prefer SN2 or the SN2’ mechanism (a cousin of SN2 that plays with the π system). Why? Their primary carbons are usually more exposed, and nucleophiles find it easier to perform a backside attack without waiting around. This makes their reactions naturally faster through SN2 pathways.
Structure and Sterics: The SN2 Advantage
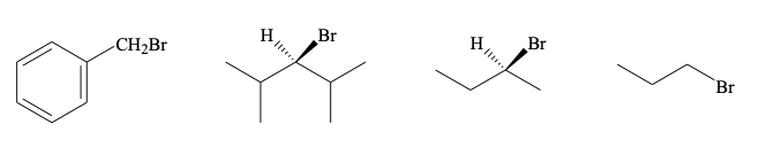
Contrary to some expectations, SN2 reactions can be faster than SN1 in many cases involving allylic or benzylic halides. Part of this is due to the nature of the carbon center being attacked.
Both benzylic and allylic positions often involve primary carbons. Primary carbons have less hindrance, so nucleophiles can easily launch a backside attack. Also, the benzene ring’s sp2 carbons are flat and provide room for the nucleophile to swoop in from above or below—making SN2 even easier.
Think of the benzene ring as a well-designed open-air stadium, where everyone can approach the star player (the electrophilic carbon) from multiple sides without bumping into obstacles. In contrast, a more crowded tertiary carbon would be like trying to reach the celebrity in a packed elevator—very tough!
Reaction Conditions: The Environmental Influencers
Whether your allylic or benzylic halide goes SN1 or SN2 depends heavily on external conditions:
- Nucleophile strength: Strong nucleophiles favor SN2 by directly attacking the electrophilic carbon.
- Solvent: Polar protic solvents stabilize carbocations and favor SN1. Polar aprotic solvents favor SN2 by letting nucleophiles be more reactive.
- Substitution level: Primary carbons are SN2 friendly; tertiary centers lean toward SN1.
Thus, it’s not just what these halides are—they’re influenced by their environment, much like how we humans behave differently in various social settings.
The Substituent Game: How Electron Donors and Withdrawers Tip the Scale
The story gets spicier when electron-rich or electron-poor groups join the party. If the benzylic halide has electron-donating groups (EDGs) on the aromatic ring, they stabilize the carbocation even more. This effect pushes the reaction mechanism towards SN1.
On the flip side, electron-withdrawing groups (EWGs) on the ring pull electron density away, destabilizing the carbocation. This discourages SN1 and leans towards SN2 mechanisms.
Scientists have plotted this switch using Linear Free Energy Relationships (LFER), essentially a graph that maps how tweaking these groups changes the reaction route. This adds a quantitative flair to predicting the mechanism’s preference.
Practical Tips: What Does This Mean for the Lab and Industry?
If you’re designing a synthesis or analyzing a reaction involving these halides, keep these tips in mind:
- Assess the substrate: Identify if the halide is benzylic or allylic and check for substituents.
- Choose your nucleophile and solvent wisely: Want an SN2 path? Go for strong nucleophiles and polar aprotic solvents.
- Consider the reaction speed: SN2 reactions tend to be faster due to fewer steps.
- Plan for substitution effects: Watch out for substituents that might stabilize the carbocation and change routes.
For example, if you have a benzylic bromide bearing a methoxy group (an EDG), expect the SN1 path to dominate. But swap that methoxy for a nitro group (an EWG), and the scale swings toward SN2.
Why Does This Matter Beyond the Textbook?
Understanding whether an allylic or benzylic halide reacts via SN1 or SN2 isn’t just academic—it leads to better yields, fewer side products, and smarter synthetic routes. Pharmaceutical companies burn less reagent and waste less time, while chemists minimize headaches.
Imagine spending hours trying to optimize a synthesis only to realize the nucleophile or solvent choice pushed your reaction the wrong way. Knowing the interplay of these factors up front saves time and money. It’s like having a reaction GPS that avoids detours.
Wrapping It Up
So, will allylic and benzylic halides prefer SN1 or SN2? The simple truth is neither chooses strictly. They walk both paths but their favored route hinges on carbocation stability, reaction conditions, and substituent effects.
Benzylic halides tend to lean towards SN1 when stabilized by electron-donating groups, while allylic halides often favor a quick SN2 or SN2’ attack, especially with primary carbons and strong nucleophiles. Conditions like solvent and nucleophile strength often tip the scales.
Next time you face this question in the lab or exam, remember: context is king, and these halides are flexible players in the molecular game.
Got your own experiences or quirky reaction tales with allylic or benzylic halides? Feel free to share. After all, chemistry is as much about stories as it is about molecules!
1. Do benzylic and allylic halides both undergo SN1 reactions?
Yes, they can both proceed via SN1 since their carbocations are stabilized. This stabilization eases the carbocation formation, a key SN1 step.
2. Which reaction does a benzylic halide prefer, SN1 or SN2?
Benzylic halides typically favor SN1 reactions more. Their carbocations gain extra stability from the aromatic ring, making SN1 pathways easier.
3. How do allylic halides react compared to benzylic halides?
Allylic halides often favor SN2 or SN2′ mechanisms. The structure permits nucleophilic attack directly, so SN2 is usually faster for these.
4. How do substituents affect the reaction pathway of benzylic halides?
Electron withdrawing groups push benzylic halides toward SN2, while electron donating groups stabilize carbocations and favor SN1 mechanisms.
5. Why is SN2 faster for primary benzylic and allylic halides?
- SN2 attacks an exposed primary carbon.
- The planar sp² structure allows nucleophiles easy access.
- This reduces steric hindrance, speeding up SN2 reactions.




Leave a Comment