How Many Resonance Structures Does Pyridine Have?
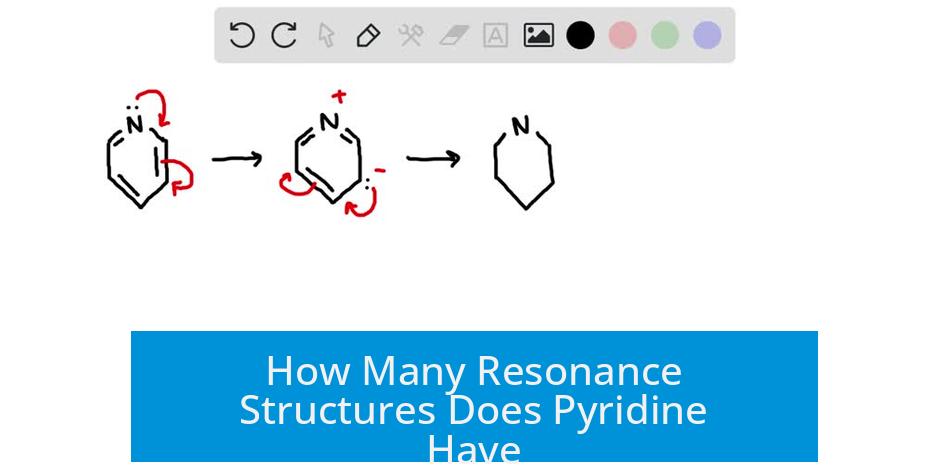
Pyridine has eight resonance structures in total, including two neutral forms and six zwitterionic ones; among these, three resonance structures contribute significantly to its resonance hybrid. This count considers the electron delocalization within the aromatic ring and the involvement of the nitrogen atom at the ring position.
Understanding Resonance Structures in Pyridine
Resonance structures illustrate different electron arrangements within a molecule without altering the placement of atoms. In pyridine, these structures show the shifting of double bonds and the lone pair on nitrogen across the six-membered ring.
- Total resonance structures: 8 (2 neutral, 6 zwitterionic)
- Significant contributors: 3 resonance forms mainly influence the molecule’s electronic structure
- Types of zwitterionic forms: 3 with positive charge on nitrogen and 3 with negative charge on nitrogen
Types of Resonance Structures in Detail
Neutral Resonance Structures
Two neutral structures resemble benzene’s resonance forms, where the nitrogen’s lone pair remains localized and does not participate in aromaticity. These forms maintain the overall neutrality of the molecule.
Zwitterionic Resonance Structures
Six zwitterionic forms exist:
- Three with a positive charge on the nitrogen atom. These have a lower contribution to the resonance hybrid because placing a positive charge on nitrogen destabilizes the molecule.
- Three with a negative charge on nitrogen. These structures result from shifting electrons such that nitrogen gains an extra electron density, producing a negative charge.
These charged forms generally contribute less to the resonance hybrid due to decreased stability relative to the neutral forms.
Equivalence and Distinctions Among Resonance Structures
Among the neutral forms, some structures may appear distinct but are considered equivalent because their double bonds are delocalized thoroughly around the ring, preserving aromaticity. Specifically, resonance structures labeled 1 and 5 (from typical resonance illustrations) are equivalent but not literally the same. This equivalence reflects the molecule’s overall symmetry and electron delocalization.
Effect of Nitrogen Atom on Resonance
Nitrogen replaces one carbon in the six-membered ring, impacting the resonance patterns compared to benzene. It contributes a lone pair that does not take part in the aromatic sextet, contrasting with five-membered heterocycles where nitrogen’s lone pair often contributes to aromaticity.
The ring maintains a planar hexagonal geometry with 120° bond angles, allowing effective overlap of p orbitals and sustaining Hückel’s aromatic electron count with six π electrons.
Charge Distribution and Electrophilic Substitution Implications
Three resonance structures exhibit positive charges on carbon atoms in positions 2 and 4 adjacent to the nitrogen. These positive charges create a partial localization of positive charge, influencing reactivity.
Due to these charges, electrophilic aromatic substitution (EAS) is hindered in pyridine, making it less reactive toward electrophiles than benzene. This behavior is essential in understanding pyridine’s chemical properties and reactivity in synthesis.
Summary Table of Pyridine Resonance Structures
| Type | Number of Structures | Charge Distribution | Contribution to Resonance Hybrid |
|---|---|---|---|
| Neutral | 2 | No formal charge; lone pair on N unaffected | Major contributors |
| Zwitterionic (Positive on N) | 3 | Positive charge on N; negative charge elsewhere | Minor contributors due to instability |
| Zwitterionic (Negative on N) | 3 | Negative charge on N; positive charge elsewhere in ring | Minor contributors |
Verifying Your Work on Pyridine Resonance
When counting resonance structures, include all possible electron shifts that maintain the aromatic sextet. The 8 structures, comprised of 2 neutral and 6 zwitterionic ones, are standard counts in organic chemistry literature.
Focus on the three most significant forms, usually neutral or close to neutral. These dominate the resonance hybrid and accurately describe the molecule’s electron distribution.
Remember, resonance forms are not equally contributing. Charged species typically have lower weights. Check for equivalency among structures to avoid double counting.
Key Points to Remember
- Pyridine shows 8 resonance structures: 2 neutral and 6 zwitterionic.
- Three resonance structures mainly contribute to the electron delocalization.
- Nitrogen’s lone pair does not participate in aromaticity but influences resonance.
- Zwitterionic forms with charges on nitrogen have lower resonance weights.
- Positions 2 and 4 in the ring often carry positive charges in zwitterionic forms.
- Equivalence of some resonance forms prevents counting duplicates.
- Electron delocalization preserves pyridine’s aromaticity similarly to benzene.
How Many Resonance Structures Does Pyridine Have? Can Someone Check My Work?
So, you’ve been scratching your head over the resonance structures of pyridine? You’re not alone. Pyridine, a six-membered aromatic ring like benzene, but with a nitrogen atom replacing one carbon, feels like the chemistry equivalent of a cool mystery novel. To answer your question directly: pyridine has 8 resonance structures in total. But, before you groan at chemistry complexity, let’s unpack what that really means and why it matters.
Pyridine keeps it interesting by mixing neutral and charged resonance forms. There are two neutral resonance structures, which are your straightforward, most stable contributors. Then, there are six zwitterionic resonance forms, split evenly between positive and negative charges on the nitrogen atom.
The Breakdown: Neutral, Zwitterionic, and Charged Structures
Among the eight resonance structures:
- Two Neutral Structures: These resemble benzene’s resonance and are the stars of the resonance show. Although structures 1 and 5 look similar, they are not identical but equivalent due to the aromatic delocalization of electrons.
- Three Zwitterionic with Positive Charge on Nitrogen: These have less impact on the overall resonance hybrid because positive charge on nitrogen is energetically less favorable. They are, in fact, poor contributors to the stability of pyridine.
- Three Zwitterionic with Negative Charge on Nitrogen: These contribute more, shifting electron density towards the nitrogen. They highlight how flexible the electron cloud is in response to different electronic demands.
- Three Resonance Structures with Positive Charge on Carbons 2 and 4: These are particularly important because they underline why electrophilic aromatic substitution (S_EAr) is less favorable for pyridine compared to benzene. The positive charge distributed on carbons dampens the ring’s reactivity.
This distribution of charges and electron density tweaks pyridine’s chemical behavior significantly compared to benzene. You can think of pyridine as the “cool cousin” of benzene who’s similar but adds a twist to the party—thanks to that nitrogen atom.
Why Does This Matter? Aromaticity and Electron Delocalization
Pyridine maintains its aromatic sextet of six π electrons, satisfying Hückel’s rule like benzene. But here’s the kicker: unlike heterocycles such as pyrrole, the lone pair on nitrogen in pyridine doesn’t jump into the aromatic π system. Instead, it sits on nitrogen, contributing to different resonance forms that affect the compound’s chemistry.
The resonance structures collectively form a hybrid, creating a molecule more stable than any single structure. This hybrid confirms that pyridine’s properties cannot be pinned down to just one drawing—it’s a dynamic dance of electrons shifting their places, giving pyridine its unique chemical personality.
Peeking Into the Chemistry Lab: Practical Implications
Understanding pyridine’s resonance helps chemists predict its reactivity, especially in electrophilic aromatic substitution reactions. The ring’s partial positive charge on carbons 2 and 4 discourages incoming electrophiles, unlike benzene, which is more welcoming to such substitutions.
Interestingly, when you look at pyridinium N-oxide—a related compound—the resonance story shifts a bit. The oxygen acts as a nucleophile and increases electron density at certain ring positions, leading to different reactivity patterns, like SN2-type shifts forming N-alkoxypyridinium salts.
So, knowing resonance contributors isn’t just an academic exercise. It’s a map guiding chemists on where reactions are likely to occur and how stable different intermediates might be.
Is 8 Resonance Structures a Hard Number? Not Always.
Here’s where it gets a tad philosophical (or at least nuanced). Sometimes, you’ll see sources say “3 major resonance structures.” That’s because among the 8 total, only a few contribute significantly to the molecule’s stability. The rest are minor players—valid but not impactful. For beginners, focusing on these three major contributors can clarify understanding before diving into the full set.
Quick Recap and How to Check Your Work
Key Points:
- Pyridine has 8 resonance structures; 2 neutral, 3 zwitterionic with positive nitrogen, 3 with negative nitrogen.
- The 2 neutral structures (1 and 5) are equivalent but distinct, reinforcing delocalized π-electrons and aromaticity.
- Charge distribution affects reactivity and stability, especially with positive charges on carbons 2 and 4.
- Focus on the 3 most significant structures if you want a simplified overview.
To check your work, sketch every resonance form keeping the nitrogen’s lone pair in mind. Verify charges make sense (no impossible charge states) and remember the resonance contributors must preserve the aromatic 6 π-electron count. If your forms violate these rules, it’s time to rework.
Final Thought: Why Should You Care?
Pyridine’s resonance structures aren’t just chemistry homework fodder. They reveal how tiny changes—switching one carbon for nitrogen—completely shake up electron flow, ring stability, and reactivity. Grasp these details, and you wield the power to predict how pyridine behaves in countless reactions from drug design to materials science.
So next time someone asks, “How many resonance structures does pyridine have?” you can confidently say, “Eight in total, with three major players shaping its chemical personality.” And if anyone wants to double-check your work, just invite them to this post—because hey, chemistry is better when shared.
How many resonance structures does pyridine have in total?
Pyridine has 8 resonance structures total. These include 3 zwitterionic forms with positive charge on nitrogen, 3 with negative charge on nitrogen, and 2 neutral structures.
How many resonance structures of pyridine are considered significant?
There are 3 significant resonance structures in pyridine. These depict electron delocalization within the ring and contribute most to the molecule’s stability.
Are all resonance structures of pyridine neutral?
No, not all are neutral. Pyridine has 2 neutral resonance forms and 6 zwitterionic forms—some with positive charge on nitrogen and others with negative charge on nitrogen.
Why are some resonance structures of pyridine zwitterionic?
Because the charges can localize on nitrogen or carbon atoms, some resonance forms show positive and negative charges separated. These zwitterionic structures usually contribute less to the overall resonance hybrid.
Are any pyridine resonance structures equivalent?
Yes, for example, structures 1 and 5 are equivalent due to delocalized double bonds across the ring, but they are not identical. This equivalence arises from the aromatic nature of pyridine.
How does nitrogen affect pyridine’s resonance compared to benzene?
Nitrogen changes resonance patterns in pyridine. While the ring remains aromatic with 6 π electrons, the nitrogen’s lone pair is not part of the aromatic sextet, altering electron distribution relative to benzene.



Leave a Comment