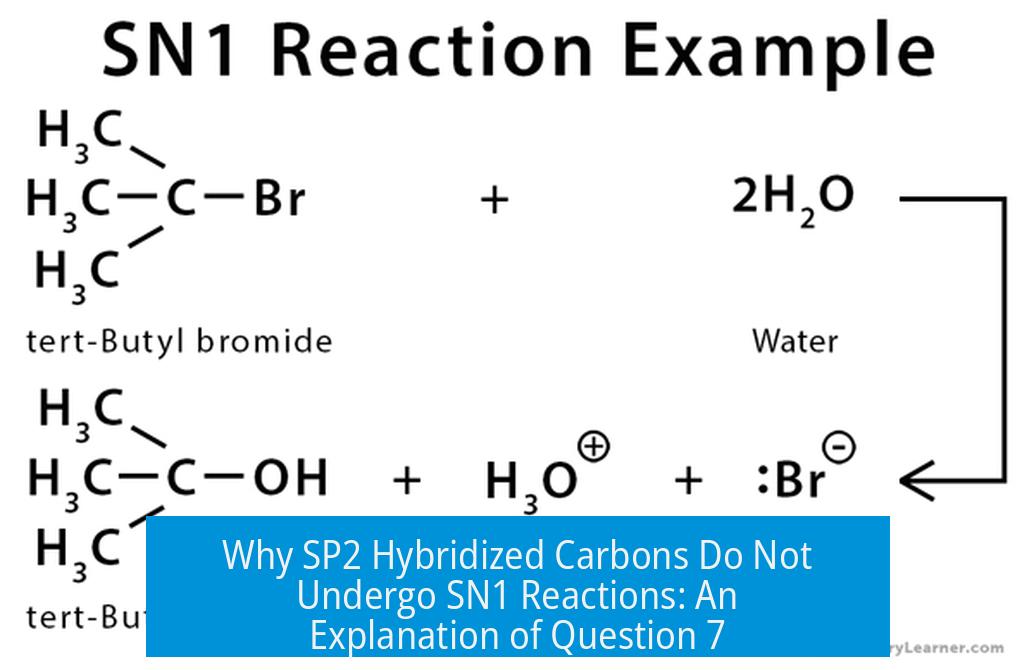
Why Can’t sp2 Hybridized Carbons Undergo SN1 Reactions? Explanation of Question Number 7

sp2 hybridized carbons rarely undergo SN1 reactions because their higher s-character makes cleavage to form carbocations energetically costly, and in rigid bicyclic systems, the geometry prevents carbocation formation essential for SN1. Specifically, bridgehead carbons, often mistaken as sp2, are sp3 and locked in a non-planar geometry, blocking the formation of the planar carbocation intermediate required for SN1 pathways.
Understanding Hybridization at Bridgehead Carbons
A common misconception is that bridgehead carbons in bicyclic systems are sp2 hybridized. In reality, bridgehead carbons are typically sp3 hybridized. This distinction is critical because SN1 reactions rely on carbocation intermediates adopting a trigonal planar geometry, which corresponds to sp2 hybridization. Since bridgehead carbons cannot adopt this planar geometry, the carbocation formation is hindered.
This misconception often arises because bridgehead carbons can be attached to multiple substituents, making them look like sp2 centers. However, the ring strain and rigidity in bicyclic systems enforce sp3 hybridization at these positions.
Geometric Constraints Preventing Carbocation Formation
- SN1 reactions proceed via carbocation intermediates that require trigonal planar, sp2 geometry.
- A carbocation intermediate has an empty p-orbital perpendicular to three substituents in the plane.
- Bridgehead carbons are locked in a rigid bicyclic framework that prevents flattening or planarization.
- Therefore, bridgehead carbons cannot stabilize carbocations because they cannot achieve the required planar geometry.
This geometric restriction agrees with Bredt’s rule, which forbids double bonds or planar carbocations at bridgehead positions in small bicyclic systems due to ring strain.
Hybridization and Electronic Considerations in SN1
SN1 involves a stepwise mechanism where the leaving group departs first to form a carbocation, then a nucleophile attacks. The stability and formation ease of the carbocation determine reaction feasibility.
- sp3 hybrid carbons with lower s-character hold electrons more loosely, easing carbocation formation.
- sp2 hybrid carbons have higher s-character (33%) compared to sp3 (25%), holding electrons more tightly.
- This makes electron removal—and thus carbocation formation—more energetically expensive at sp2 carbons.
Consequently, while SN1 reactions can theoretically occur at some sp2 carbons, these cases are rare and energetically unfavorable.
Steric Hindrance and Its Role in Substitution Reactions
Question number 7 highlights the stereochemical and steric issues with SN1 and SN2 at bridgehead carbons.
- SN2 reactions struggle at tertiary carbons, including bridgehead ones, due to steric hindrance. Bulky groups crowd the nucleophile’s attack path.
- SN1 reactions require planar carbocation intermediates. The bicyclic ring locks bridgehead carbons in a fixed conformation, preventing planarity.
- Thus, albeit SN1 might be minorly feasible, steric factors and geometry reduce its rate or make it impractical.
The bicyclic system’s rigidity not only blocks planar carbocation formation but also maintains steric congestion. This combination disfavors both SN1 and SN2 at bridgehead carbons.
Bredt’s Rule and Carbocation Stability
Bredt’s rule explains why bridgehead carbocations or double bonds cannot form in small bicyclic rings. This rule states that double bonds cannot exist at bridgehead positions unless the ring system is large enough to relieve strain.
An analogous strain applies to carbocations requiring planar geometry at the bridgehead carbon. The induced strain to flatten this carbon is too high, so carbocation formation—and thus SN1—is not observed.
Exceptions and Special Cases
Though uncommon, some substitution reactions occur at sp2 carbons or bridgehead positions under special conditions.
- Friedel-Crafts alkylation, a Lewis acid-catalyzed substitution, can proceed with certain bridgehead substrates by stabilizing the carbocation differently.
- Substitutions at sp2 carbons often proceed through addition-elimination mechanisms rather than pure SN1.
- Such cases demonstrate that while SN1 at sp2 or bridgehead carbons is highly unfavorable, it is not absolutely impossible.
Summary Table: SN1 Feasibility vs. Carbon Hybridization and Structure
| Carbon Type | Hybridization | Carbocation Geometry | SN1 Feasibility | Reason |
|---|---|---|---|---|
| Regular alkyl carbon (primary, secondary) | sp3 | Planar carbocation can form | Favorable | Less steric hindrance, carbocation stable enough |
| Tertiary alkyl carbon | sp3 | Planar carbocation can form | Very favorable | Carbocation highly stabilized by alkyl groups |
| Bridgehead carbon in bicyclic system | sp3 (not sp2) | Carbocation formation blocked | Not favorable | Geometry prevents planar carbocation (Bredt’s rule) |
| sp2 carbon (e.g., vinyl, aryl) | sp2 | Planar carbocation possible | Rarely favorable | High energy cost, electrons tightly held |
Key Takeaways
- Bridgehead carbons are sp3 hybridized, not sp2, and cannot adopt planar geometry needed for SN1 carbocation intermediates.
- Rigid bicyclic rings prevent bridgehead carbons from flattening, blocking carbocation formation.
- sp2 carbons have higher s-character, making SN1 energetically unfavorable due to stronger electron holding.
- Steric hindrance inhibits SN2 at tertiary and bridgehead carbons, while geometric constraints hinder SN1.
- Bredt’s rule explains why carbocations or double bonds cannot form at bridgehead positions in small rings.
- Some exceptional reactions may involve substitution at bridgehead carbons, but SN1 remains highly disfavored.



Leave a Comment