Benzil Benzilic Acid Rearrangement: Which Group Migrates, Ph or H?

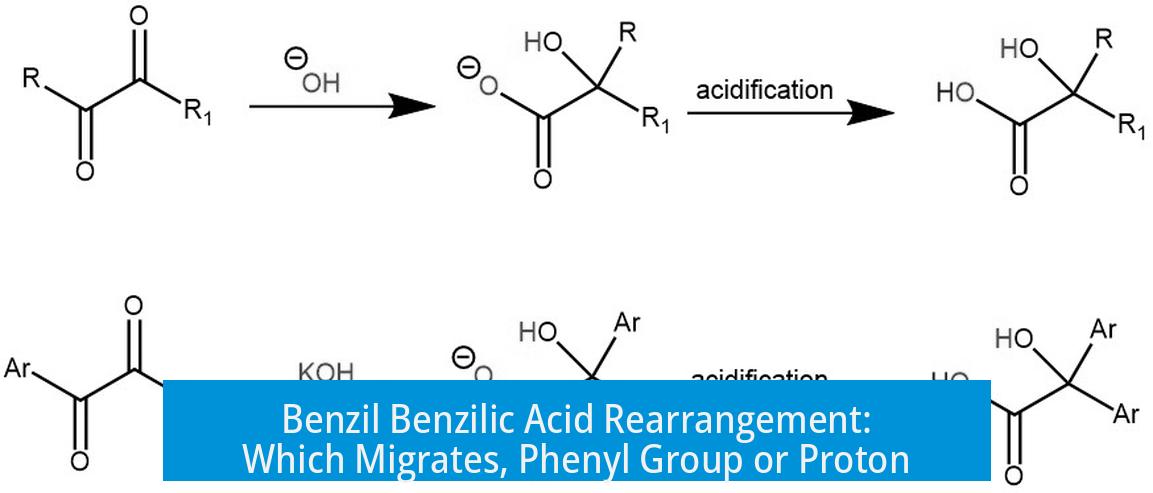
In the benzil benzilic acid rearrangement, the migrating group is the phenyl group (Ph), not the proton (H). This reaction specifically features a 1,2-shift of the substituent attached to the carbonyl carbon attacked by hydroxide.
Overview of the Reaction
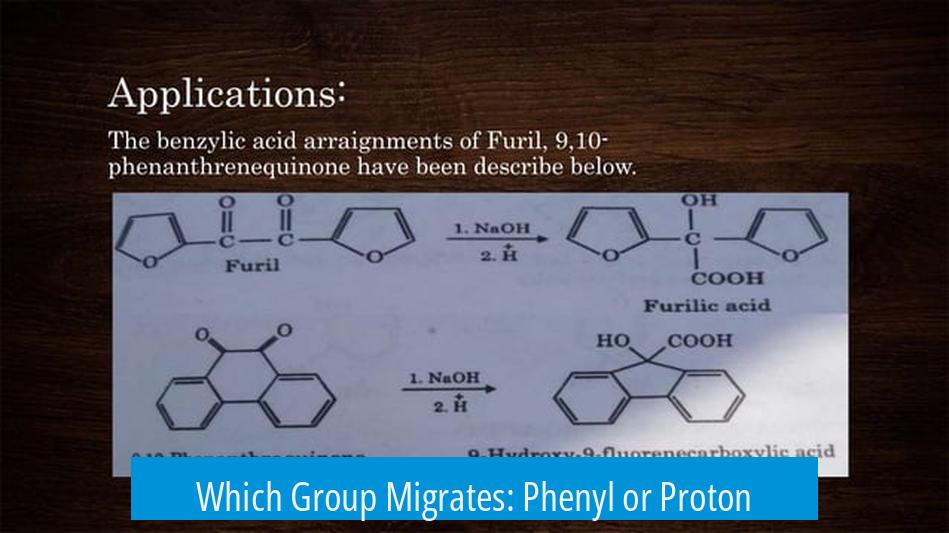
The benzil benzilic acid rearrangement transforms benzil, a 1,2-diketone where R and R’ are phenyl groups, into benzilic acid salt under basic conditions. The reagent commonly used is an alkali hydroxide, which provides the nucleophile hydroxide ion (OH−). The reaction happens in a basic environment, necessary for nucleophilic attack and subsequent rearrangement steps.
Mechanism Detail: Migration During Rearrangement
- Hydroxide ion attacks one of the two carbonyl carbons in benzil, forming a tetrahedral alkoxide intermediate.
- The substituent group (R), which in benzil is a phenyl group (Ph), migrates with its bonding electrons from the attacked carbonyl carbon to the adjacent carbon (a 1,2-shift).
- This migration does not involve simple proton (H) relocation but the movement of the entire substituent bonded to the carbonyl.
- After migration, proton transfer occurs to form the final carboxylate product (benzilate ion) and subsequently benzilic acid salt upon acidic workup.
Which Group Migrates: Phenyl or Proton?
In this reaction, the migrating group is the substituent attached to the attacked carbonyl carbon. For benzil, this substituent is phenyl (Ph). The proton (H) remains static; it plays no role in the migration step.
Hence, the key migration is:
| Group | Migrates? |
|---|---|
| Phenyl (Ph) | Yes |
| Proton (H) | No |
Role of pH
The reaction requires a high pH (basic) environment. Alkali hydroxide generates the nucleophilic hydroxide, but pH does not participate as a migrating group. The pH influences the reaction conditions but does not affect which group migrates.
Key Takeaways
- The benzil benzilic acid rearrangement involves a 1,2 migration of the substituent attached to the carbonyl carbon attacked by hydroxide.
- For benzil, the substituent is phenyl (Ph), so the phenyl group migrates.
- The proton (H) does not migrate during the rearrangement step.
- The reaction occurs under basic conditions, where hydroxide acts as the nucleophile.
Which group migrates during the benzil benzilic acid rearrangement, phenyl (Ph) or hydrogen (H)?
The phenyl group (Ph) migrates during the rearrangement. The migration involves the substituent attached to the carbonyl carbon where the nucleophile attacks. Hydrogen does not migrate in this reaction.
Why does the phenyl group migrate rather than hydrogen in this rearrangement?
The migration occurs as a 1,2 shift of the substituent R bonded to the attacked carbonyl carbon. In benzil, this substituent is phenyl, which is more likely to migrate due to its bonding. Protons do not participate in this migration step.
Does the nucleophile (hydroxide) migrate during the benzilic acid rearrangement?
No, the hydroxide ion initiates nucleophilic attack but does not migrate. Migration is limited to the substituent group attached to the carbonyl carbon, such as the phenyl group in benzil.
How does pH affect the benzilic acid rearrangement and the migrating group?
The reaction requires a basic environment to generate the hydroxide ion nucleophile. The migration step is independent of pH, involving the phenyl group shift, not proton migration.
Can the migrating group in benzilic acid rearrangement be something other than phenyl?
Yes. The migrating group corresponds to the substituent R bonded to the carbonyl carbon where hydroxide attacks. This can vary depending on the diketone used, but in benzil, it is phenyl.




Leave a Comment