Reaction Shortcuts: Understanding and Applying Key Reagents
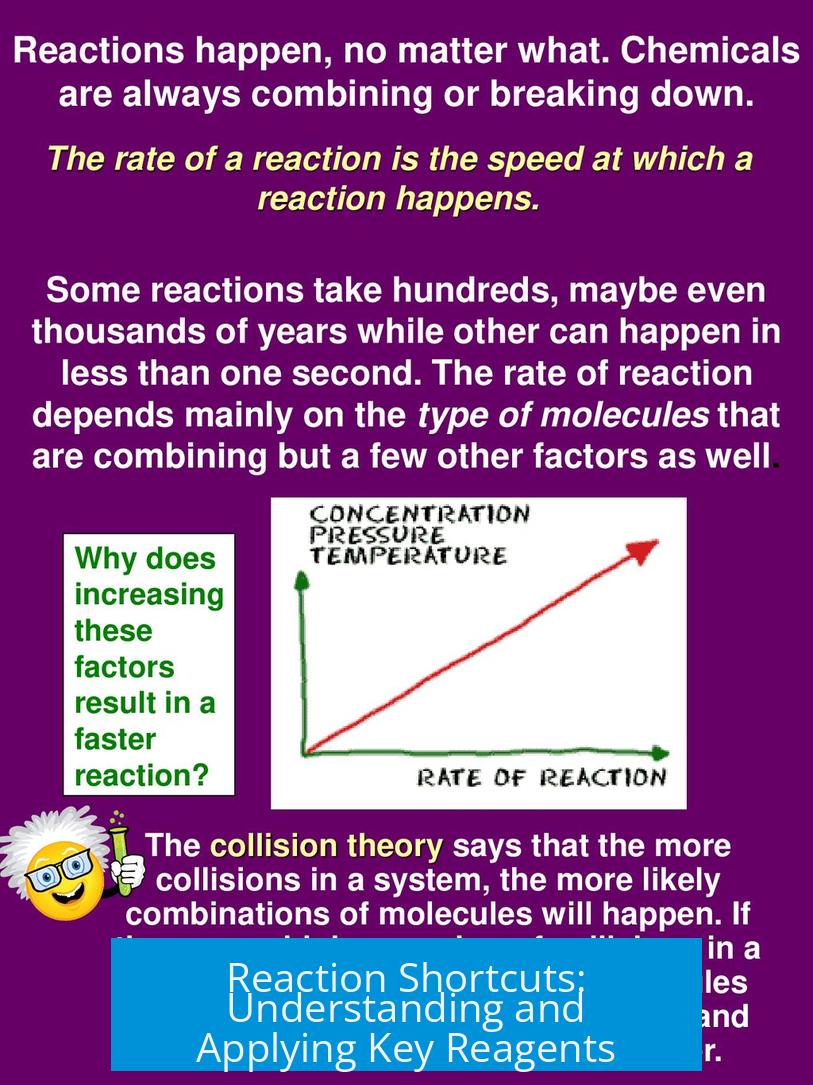
Reaction shortcuts revolve around recognizing the roles of reagents to predict their behavior efficiently. Instead of rote memorization, grasping the chemical function and mechanism behind reagents allows for streamlined problem solving in organic chemistry.
Key Reagents and Their Roles
- NaNH2 (Sodium amide): It acts as a strong base, typically deprotonating acidic hydrogens. This basic property is central to predicting its reactions, such as forming carbanions or activating substrates for nucleophilic substitutions.
- MeI (Methyl iodide): Functions as an alkyl halide and electrophile. It is commonly used as an alkylating agent, adding methyl groups by reacting with nucleophiles.
Identifying these general roles helps chemists anticipate the outcome of reactions without memorizing specific transformations.
Why Mechanistic Understanding Matters
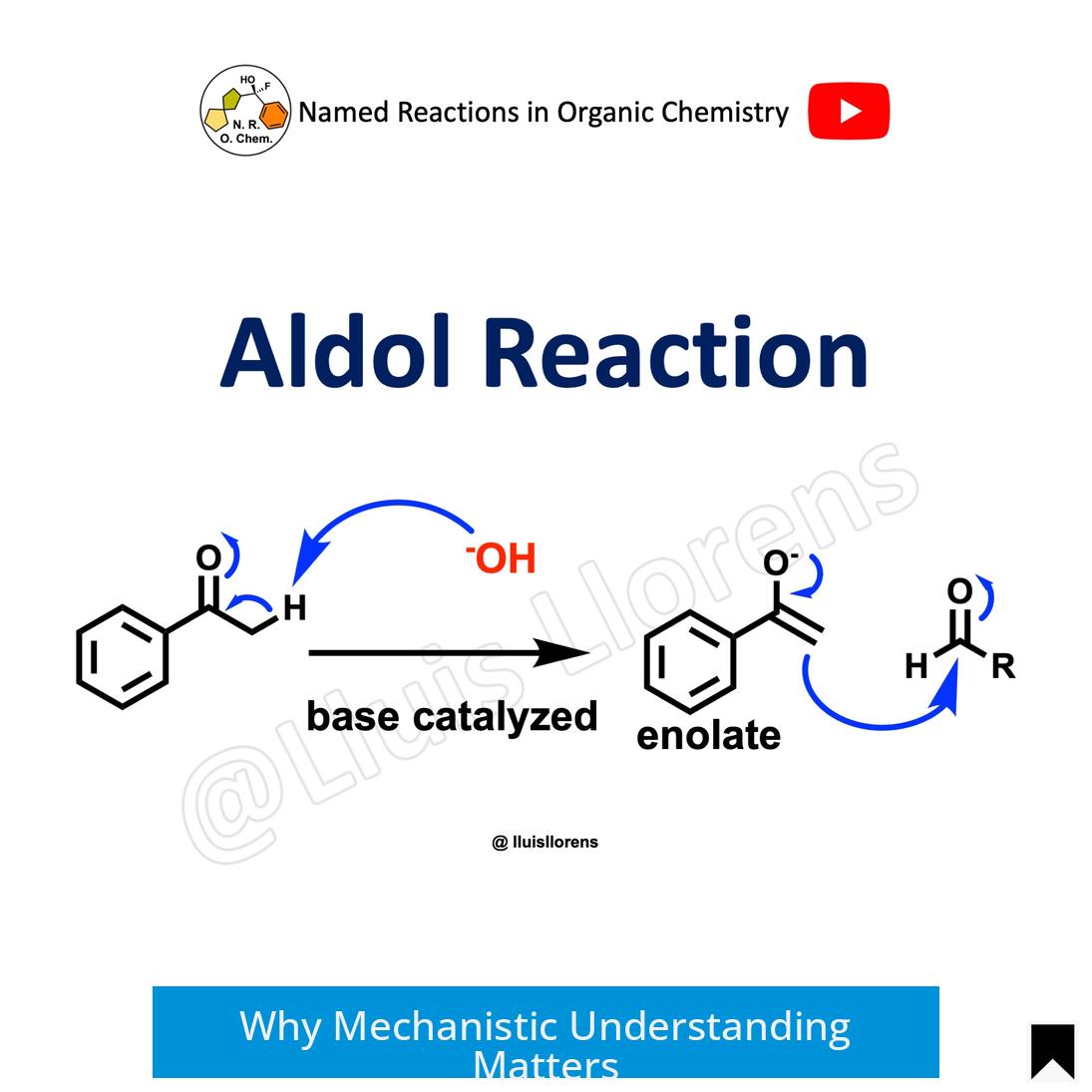
Knowing reaction mechanisms is crucial. When the mechanism is clear, the chemical nature of reagents becomes intuitive, enabling predictions of their functions. This approach emphasizes understanding the nature of molecules instead of relying on isolated facts.
For example, viewing sodium amide purely as a base, and methyl iodide as an electrophile, aligns with their reactivity profiles. This mindset facilitates problem-solving, thus reducing cognitive load during exams or synthesis planning.
Learning Through Chemical Logic
Learning reagent behavior through general functional roles rather than memorizing outcomes enhances retention and application. Understanding that NaNH2 removes protons while MeI donates alkyl groups enables quick inference of product structures from reactant combinations.
This framework empowers students to reason through unfamiliar problems and design synthetic routes effectively.
Practical Strategies to Master Reaction Shortcuts
- Focus on reagent classification: base, electrophile, nucleophile, oxidant, reductant.
- Study mechanisms to connect reagent properties to reaction pathways.
- Practice diverse reaction problems regularly for reinforcement.
- Apply shortcuts in retrosynthesis to streamline planning.
Repetition solidifies these shortcuts into automatic reasoning, reducing reliance on memorization.
Key Takeaways
- Reaction shortcuts depend on understanding reagents’ functional roles.
- NaNH2 is a strong base that deprotonates acidic sites.
- MeI acts as an electrophilic alkylating agent.
- Mechanistic knowledge enhances prediction beyond memorization.
- Regular practice is essential for ingraining shortcuts.




Leave a Comment