E2 Antiperiplanar Configuration Explained
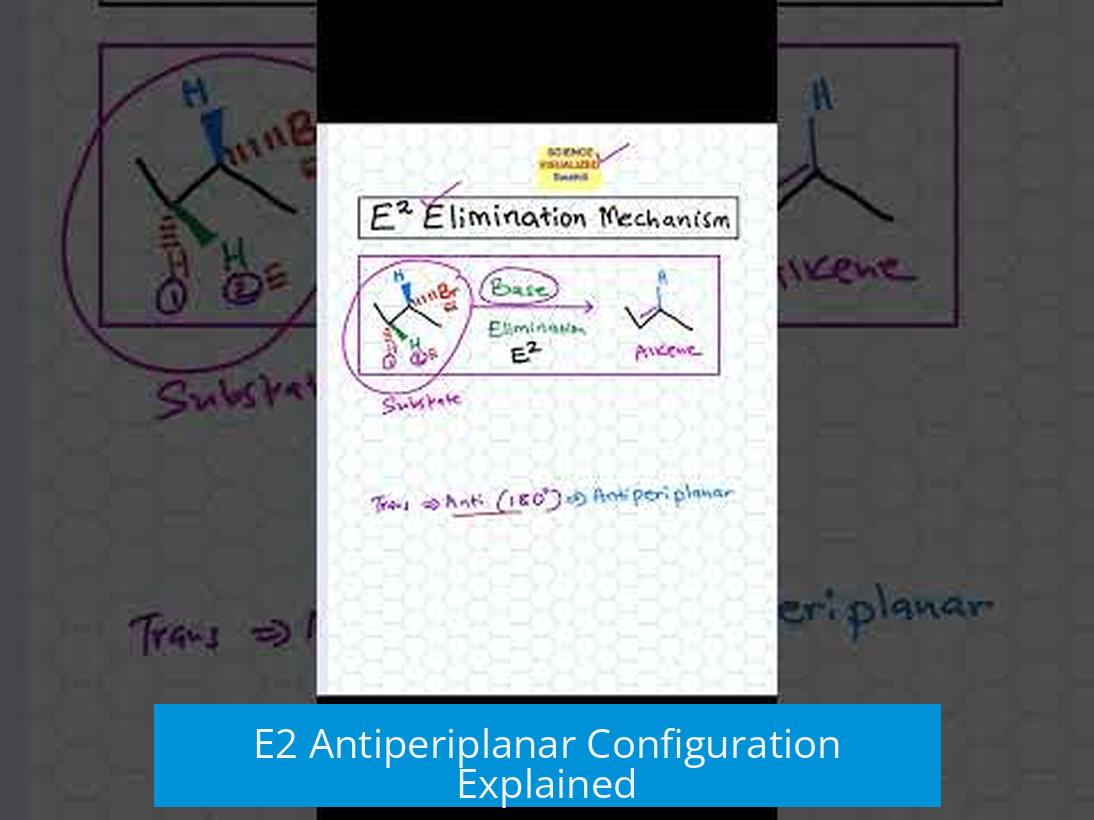
The E2 elimination reaction requires the leaving group and the beta hydrogen to be antiperiplanar—180° apart in the same plane—to achieve proper orbital overlap that stabilizes the transition state and allows the concerted breaking of bonds forming an alkene.
Role of Antiperiplanar Positioning in E2
The E2 mechanism proceeds in a single step. Both the removal of the beta hydrogen and the departure of the leaving group happen simultaneously. The antiperiplanar arrangement means the hydrogen beta to the leaving group and the leaving group itself lie opposite each other in the same plane.
This positioning is critical because it allows orbitals to overlap effectively during the transition state. Without this alignment, the elimination does not proceed efficiently.
Orbital Interactions During the Transition State
In an antiperiplanar setup, the sigma bond of the C-H that breaks aligns opposite to the sigma antibonding (σ*) orbital of the C-X (carbon-leaving group) bond.
- When the base abstracts the beta hydrogen, electron density shifts into the σ* orbital of C-X.
- This transfer weakens and breaks the C-X bond.
- The simultaneous cleavage of C-H and C-X bonds leads to forming the C=C pi bond, producing an alkene.
Why Syn Elimination is Rare in E2

Syn elimination means the beta hydrogen and leaving group are on the same side (synperiplanar). This is uncommon because it offers less effective orbital overlap and less favorable transition state stabilization.
However, syn elimination can occur if there is no beta hydrogen in the antiperiplanar position. In such cases, the system resorts to syn elimination to proceed with the reaction.
Summary of Key Points
- E2 elimination relies on the leaving group and beta hydrogen being antiperiplanar for efficient reaction progress.
- The antiperiplanar configuration allows simultaneous breaking of C-H and C-X bonds by orbital overlap between the breaking C-H sigma bond and the antibonding C-X sigma* orbital.
- Syn elimination occurs only as a rare alternative when antiperiplanar hydrogens are absent.
Unlocking the Mystery of E2 Antiperiplanar Configuration: Why Geometry Rules the Reaction
What exactly is the E2 antiperiplanar configuration? In the simplest terms, it refers to the ideal geometric relationship between the leaving group and the beta hydrogen in an E2 elimination reaction—where these two groups are positioned 180 degrees apart in the same plane. This alignment is not just a quirky preference; it’s the KEY to making the reaction happen smoothly and efficiently.
Imagine the E2 reaction as a choreographed dance where the leaving group (like bromine or chlorine) and the beta hydrogen must stand opposite each other to perform the perfect exit move. This antiperiplanar arrangement ensures that the orbitals involved—think of these as dance partners—overlap just right to stabilize the reaction’s high-energy transition state.
The Science Behind the Scenes: Orbital Overlap in Action

The E2 mechanism is a single-step, concerted process. This means the proton abstraction by the base and the leaving group’s departure happen simultaneously. The “secret sauce” lies in how the electrons are shuffled during this event.
Here’s what’s cool: when the beta hydrogen’s C-H sigma bond breaks, the electrons from that bond don’t just vanish. They slide over into the sigma antibonding orbital (sigma*) of the C-X bond (where X is the leaving group). This overlap weakens the C-X bond at just the right moment, causing it to break as the new pi bond (the alkene) forms.
Without that antiperiplanar positioning, the orbital overlap is misaligned. The smooth transfer of electron density falters, making the reaction slower or favoring other mechanisms. So geometry isn’t just academic—it’s mechanistic magic.
Why Not Syn Elimination? The Rare Outlier
You might ask, “What if the hydrogen and leaving group are on the same side?” This is called syn elimination and it is a rare breed in E2 reactions.
Syn elimination can happen but usually when no antiperiplanar beta hydrogens are around. The reason it’s uncommon is clear: the antiperiplanar setup is energetically favored. It provides superior orbital alignment that syn elimination simply can’t match.
Think of it like trying to dance perfectly with someone standing directly opposite you versus standing shoulder to shoulder. The first is elegant and efficient. The second? Awkward and rare.
Practical Perspective: Why You Should Care
Chemists love the E2 reaction for its ability to create alkenes efficiently. But what makes this reaction reliable? The antiperiplanar arrangement is a key player.
When running syntheses in the lab, predicting or controlling which hydrogen will be abstracted can mean the difference between getting the desired product or an unwanted side reaction. Recognizing that only hydrogens antiperiplanar to the leaving group participate helps chemists design molecules or reaction conditions to favor the right elimination path.
For example, in cyclic compounds, the ring’s rigidity forces hydrogens into certain positions. Here, only hydrogens antiperiplanar to the leaving group can be eliminated, often influencing the stereochemical outcome of the product.
Let’s Recap: E2 and Its Geometry-Driven Dance
- E2 elimination depends on the leaving group and beta hydrogen being antiperiplanar for orbital overlap and transition state stabilization.
- This arrangement facilitates electron flow from the broken C-H bond into the antibonding orbital of the C-X bond, allowing simultaneous bond breaking and formation of an alkene.
- Syn elimination is the exception, not the rule, happening mainly if no antiperiplanar hydrogens are accessible.
Putting it simply: the antiperiplanar configuration is the golden rule of the E2 elimination mechanism, dictating not only if the reaction will proceed smoothly but also influencing the stereochemistry of the product.
Did You Know? A Myth Debunked
It’s a common misconception that any hydrogen next to the leaving group can be abstracted in an E2 reaction. Actually, only those hydrogens perfectly aligned antiperiplanar contribute effectively. This explains why sometimes a seemingly available hydrogen just doesn’t participate.
Next time you look at a molecule and wonder why elimination isn’t happening as expected, check if your beta hydrogens line up in that antiperiplanar fashion. Geometry can be a party stopper or a green light!
In Closing: A Small Twist Creates Big Impact
The E2 elimination is a reminder that in chemistry, small spatial differences matter immensely. Understanding the antiperiplanar configuration not only helps you predict reaction outcomes but also offers a peek into the elegant choreography of molecular transformations.
Isn’t it fascinating that a subtle 180-degree twist in a molecule’s shape can dictate whether an alkene forms or not? Molecular geometry runs the show, and with antiperiplanar configuration, it’s got a winning script for the E2 mechanism.
What is the role of antiperiplanar configuration in the E2 mechanism?
In E2, the leaving group and beta hydrogen must be antiperiplanar for effective orbital overlap. This positioning stabilizes the transition state and allows the elimination to proceed in a single concerted step.
How does orbital interaction occur during E2 elimination with antiperiplanar arrangement?
The C-H sigma orbital aligns antiperiplanar to the C-X sigma* antibonding orbital. Removing the beta hydrogen shifts electron density into the sigma* orbital, breaking the C-X bond and forming a new pi bond.
Why is syn elimination rare in E2 reactions?
Syn elimination occurs when the beta hydrogen and leaving group are on the same side. It is rare because antiperiplanar geometry offers better orbital overlap and lower energy transition states, preferred during E2.
Can E2 elimination occur without an antiperiplanar hydrogen?
Yes, but only through syn elimination, which is less favorable. Syn elimination typically happens only when no antiperiplanar hydrogens are available for elimination.
What does antiperiplanar mean in the context of E2 elimination?
Antiperiplanar refers to two groups being 180° apart in the same plane. For E2, it means the beta hydrogen and leaving group are opposite each other, allowing proper orbital overlap for elimination.




Leave a Comment