Understanding Thick and Dashed Lines in Organic Chemistry
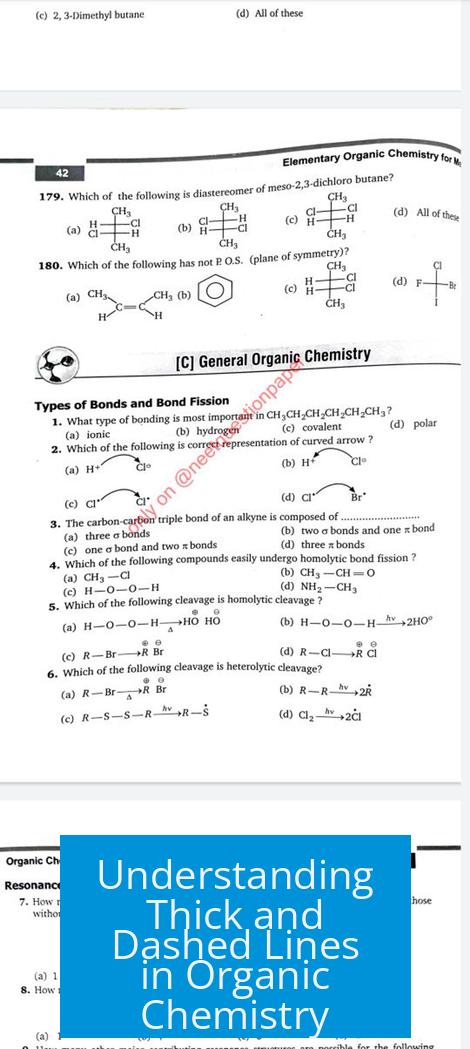
The thick line and the dashed line have distinct meanings in organic chemistry. The thick (solid) wedge shows a bond coming out of the plane of paper towards the viewer, while the dashed wedge represents a bond going behind the plane, away from the viewer. A plain dashed line, however, typically denotes delocalized bonds in resonance structures, not spatial orientation.
Thick (Solid) Wedge
The thick wedge is used to depict bonds projecting towards the observer. This helps visualize molecules in three dimensions. It clarifies which atoms or groups are pointing “out” of the flat page or screen. Such notation is key for understanding stereochemistry and the spatial layout of atoms.
Dashed Wedge
The dashed wedge is the counterpart of the thick wedge. It indicates bonds receding behind the plane of the paper. This visual cue complements the thick wedge by showing atoms oriented away from the viewer. Together, these wedges represent molecular 3D shapes clearly.
Dashed Line without a Wedge
Unlike wedges, a plain dashed line usually does not indicate 3D orientation. Instead, it often denotes delocalized bonds within resonance structures. For example, in molecules like carbonate ions, dashed lines highlight the shared or shifting nature of electrons across multiple atoms.
It is important to note that some textbooks mistakenly use plain dashed lines where dashed wedges should appear. This causes confusion for learners about molecular geometry.
Why the Difference Matters
- Correct use of wedges (thick and dashed) shows real 3D structure of molecules.
- Dashed lines mark resonance and electron delocalization, not spatial positioning.
- Mistakes in notation may confuse the molecular shape and bonding understanding.
Additional Learning Resources
For newcomers, the book Organic Chemistry as a Second Language is highly recommended. It explains these notations clearly and supports many standard textbooks well.
Key Takeaways
- Thick wedge: bond towards the viewer (out of the paper).
- Dashed wedge: bond away from the viewer (behind the paper).
- Dashed line: usually signifies delocalized bonds in resonance, not 3D orientation.
- Misuse of dashed lines instead of dashed wedges exists in some textbooks.
- Understanding these symbols is crucial for grasping molecular 3D structures and bonding.




Leave a Comment