Lewis Structures: Distinguishing Atoms Connected to the Central Atom vs. Outer Atoms
Determining whether an atom, such as hydrogen, is directly connected to a central atom or to an outer atom in Lewis structures (for example, comparing HCO2- and HCO3-) requires careful application of fundamental bonding principles, the octet rule, oxidation states, and knowledge of standard ion structures.
Fundamentals of Drawing Lewis Structures
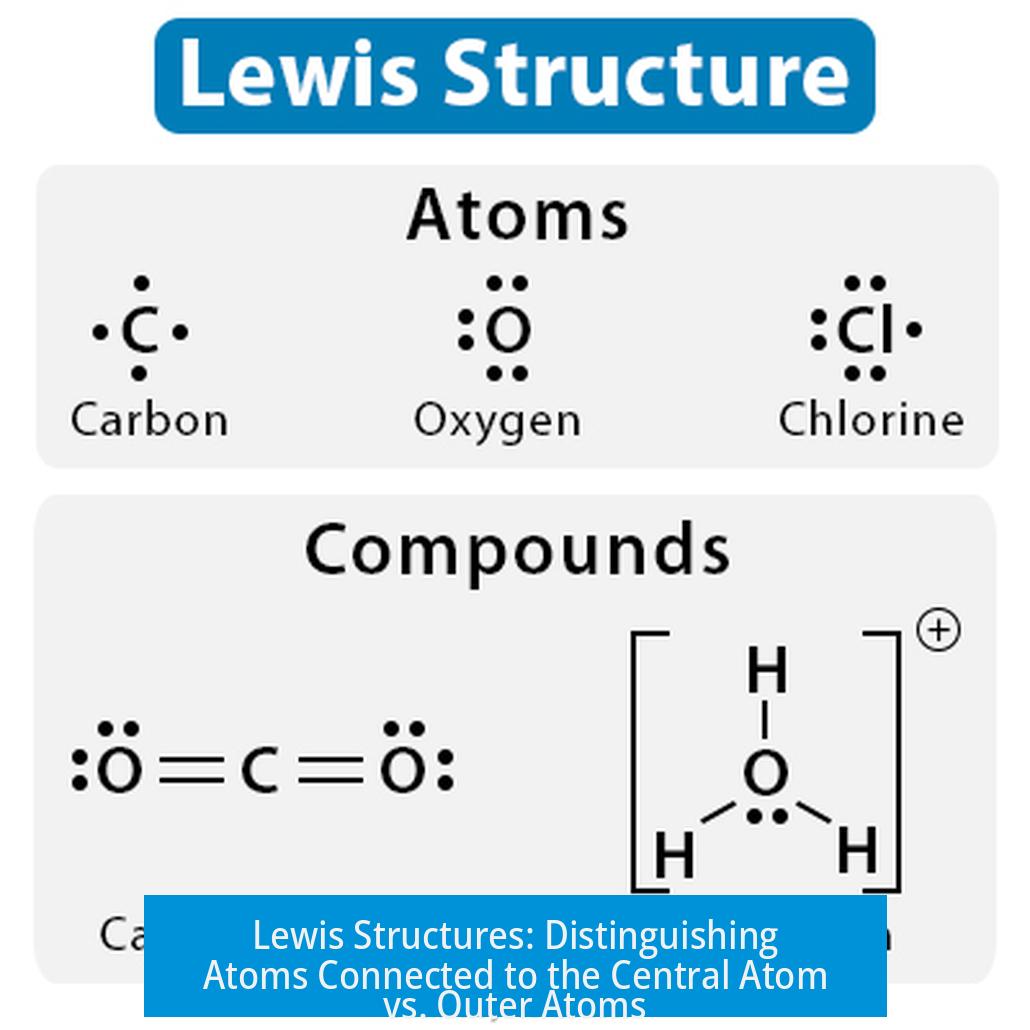
Constructing Lewis structures begins with understanding how atoms share or transfer electrons to achieve stable electron configurations. Each atom seeks to fulfill the octet rule (eight electrons in its valence shell) except hydrogen, which follows the duet rule.
In many basic general chemistry contexts, simplified rules guide Lewis structure drawing. However, these rules may not address complex polyatomic ions. To solve the connectivity question, one should explore various hypothetical bondings by drawing alternate Lewis structures.
- Draw the ion with hydrogen bonded to different atoms (central vs. outer) and examine the viability.
- Check for reasonable formal charges and overall stability in each structure.
- Eliminate structures failing the octet rule or showing destabilizing features.
Applying the Octet Rule and Oxidation States
The octet rule acts as a filter for plausible structures. Most atoms (like carbon and oxygen) strive for a full octet. Hydrogen bonds only once, forming a duet.
Oxygen nearly always exhibits a -2 oxidation state, except in peroxides or other special cases. This knowledge limits which oxygen atoms may bond to hydrogen. For example, in bicarbonate (HCO3-), hydrogen is bonded to oxygen, which aligns with oxygen’s typical oxidation state and bonding patterns.
| Ion | Hydrogen Location | Central Atom | Outer Atom(s) |
|---|---|---|---|
| HCO2- (Formate) | Bonded to central carbon | Carbon | Oxygen atoms |
| HCO3- (Bicarbonate) | Bonded to outer oxygen | Carbon | Oxygen (with bonded H) |
Structures violating these oxidation state considerations, e.g., hydrogen bonded directly to oxygen with incompatible oxidation states or carbons with incomplete octets, are unlikely to represent correct bonding.
Recognizing Known Ions and Their Standard Structures
Some polyatomic ions have widely accepted canonical Lewis structures. For example, the bicarbonate ion (HCO3-) is routinely drawn with hydrogen bonded to one of the oxygen atoms, not directly to the carbon.
Familiarity with such standard structures streamlines the identification of central versus outer atom connections. It is valuable to memorize these common ions and their Lewis structures to avoid potential errors.
Example: HCO2- vs. HCO3-
The formate ion (HCO2-) contains two oxygens bonded to carbon, with hydrogen bonded to carbon directly. Carbon is the central atom here. This arrangement satisfies the octet for carbon and typical oxidation states.
In contrast, bicarbonate ion (HCO3-) includes three oxygens with carbon central. Hydrogen bonds to one oxygen, an outer atom. This bonding follows expected oxidation states and the octet rule more comfortably than bonding hydrogen directly to carbon.
Testing alternative structures, such as bonding hydrogen to carbon in bicarbonate, results in violation of expected oxidation states and often unfavorable formal charges, making such options unlikely.
Using Structural Experimentation and Constitutional Isomers
One method to determine bonding is to sketch possible constitutional isomers, where hydrogen bonds differ. For ions with multiple potential bonding sites, comparing Lewis structures helps assess which is most stable.
- Draw structures with hydrogen on alternate atoms.
- Calculate formal charges on each atom to assess stability.
- Analyze whether atoms meet octet requirements.
For example, ethanol and dimethyl ether share the same molecular formula but differ by where hydrogen and oxygen bond, influencing their structure and properties. Similarly, forming ‘peroxy’ analogues of formate demonstrates how different placements of hydrogen and bonds affect overall structure.
Summary of Key Steps to Identify Atom Connectivity in Lewis Structures
- Apply the octet rule: Ensure all atoms except hydrogen have eight valence electrons.
- Look at oxidation states: Oxygen usually has a -2 state. Deviations are uncommon outside special cases.
- Draw alternate Lewis structures: Place hydrogen and other atoms differently, then assess stability.
- Compare formal charges: Favor structures with minimal formal charges and charge separation.
- Use known standard structures: Reference well-documented ions like bicarbonate for accuracy.
Key Takeaways
- The central atom is typically carbon in these ions; outer atoms are usually oxygen.
- Hydrogen bonds may attach to central or outer atoms, but the octet and oxidation states guide correct placement.
- Alternate bondings can be tested by drawing Lewis structures and evaluating formal charges and electron counts.
- Memorizing common polyatomic ion structures like bicarbonate helps identify atom connectivity quickly.
- Understanding Lewis structures goes beyond simplified rules; experimenting with possible structures clarifies bonding.
Lewis Structures: How to Determine if an Atom Is Directly Connected to a Central Atom or to an Outer Atom? (HCO2- vs. HCO3-)
So, you’ve got a Lewis structure puzzle on your hands. Is that hydrogen hugging the central atom, or is it cozying up to an outer atom? This question comes up a lot, especially with tricky ions like HCO2- (formate ion) versus HCO3- (bicarbonate ion). Don’t worry—we’ll clear the fog. In fact, the best way to know if an atom is directly connected to a central atom or an outer atom is to start at the basics, apply the octet rule and oxidation states, and then explore alternative structures and ion knowledge.
What’s the Big Deal About Atom Connections?
In chemistry, knowing which atoms attach where in a molecule is like figuring out who’s sitting next to whom at the dinner table. It affects the molecule’s shape, reactivity, and even taste. (Well, maybe not taste. But definitely function!) With polyatomic ions like bicarbonate versus formate, that hydrogen placement shifts the game completely.
Before we dig into detailed examples, here’s a quick sanity check: if you guessed the hydrogen connects to the central carbon in formate, you’re on the right track. For bicarbonate, that hydrogen snuggles an oxygen, not carbon. But why?
Start from the Ground Up: Fundamentals of Lewis Structures
Luckily, you don’t need a PhD to follow this. Start by reviewing the fundamentals of Lewis structures.
- Central Atom Identification: Usually, the carbon atom in organic ions acts as the central atom. Carbon makes four bonds, so it’s often the centerpiece for bonding.
- Outer Atoms: Oxygen and hydrogen typically hang out on the edges. Oxygen usually forms two bonds, hydrogen only one.
Now, try drawing your structure multiple ways. Imagine sticking hydrogen on carbon—does it make sense? If it leaves carbon with too many bonds or oxygen shortchanged, rethink. This process is called structural experimentation. It’s like molecular improvisation. Play around until you get a structure obeying the known chemical “laws.”
The Octet Rule and Oxidation States: Your Best Friends in Bonding Mysteries
The octet rule says atoms want eight electrons in their outer shell—no more, no less. If your structure gives oxygen five bonds or odd electrons, you’ve got a rebel structure that probably won’t hang out in chemistry textbooks. Oxygen has a near-universal oxidation state of -2. If this changes wildly in your drawn structure, that’s a red flag.
Example: In the formate ion (HCO2-), the hydrogen bonds to carbon. Carbon bonds to two oxygens: one with a double bond and the other bonded to hydrogen with a single bond. This respects octet and oxidation state rules.
Contrast that with bicarbonate ion (HCO3-): here, hydrogen bonds to an oxygen, not carbon. The central carbon bonds to three oxygens, one of which shares the hydrogen. This arrangement respects the octet rule, and the oxidation states work out.
Know Thy Common Ions: Memorization Without Tears

It might sound boring to memorize common ions, but it’s smart. Knowing standard structures saves you time when faced with puzzles like HCO2- or HCO3-. You don’t have to reinvent the wheel every time.
Here’s a quick cheat sheet:
- Formate (HCO2-): Hydrogen bonded to carbon.
- Bicarbonate (HCO3-): Hydrogen bonded to oxygen.
With that, you can spot differences quickly and confidently.
Testing the Waters: Drawing Constitutional Isomers
Want to double-check your hydrogen placement? Draw constitutional isomers. These are molecules with the same formula but different bond placements. For example, try swapping the hydrogen bond in bicarbonate to carbon instead of oxygen. Can you draw a valid structure without breaking octet or oxidation rules? Usually, you can’t—confirming your original structure and hydrogen placement.
If you can’t create a reasonable isomer, your original Lewis structure is most likely the way nature intended.
Real-World Chemistry: Why Does It Matter?
You might wonder, “Does this really affect me?” It does! Knowing exact bonding affects how molecules react, how enzymes recognize substrates, and how drugs function. For example, bicarbonate plays a critical role in blood pH buffering—its structure influences how it interacts within your body.
Accurate Lewis structures help chemists predict & manipulate reactions in fields from pharmaceuticals to environmental science.
Summary: Your Step-by-Step Cheat Sheet
- Identify the Central Atom: Usually the atom that forms the most bonds (often carbon).
- Apply the Octet Rule: Check that all atoms satisfy octet or duet (for hydrogen) without odd electron counts.
- Consider Oxidation States: Oxygen is nearly always -2; carbon varies but follow usual rules.
- Compare Known Structures: Memorize structures of common ions like bicarbonate and formate.
- Experiment: Draw alternative isomers; if none fit, original bonding is correct.
Final Thoughts
In chemistry, you’re a detective with a molecular magnifying glass. By focusing on basic principles, testing alternatives, and knowing reliable structures, you can confidently distinguish if an atom is connecting to the central atom or an outer atom. For tricky ions like HCO2- and HCO3-, this approach works like a charm.
So, next time you stare at a Lewis structure wondering, “Is that hydrogen on carbon or oxygen?”—remember: fundamentals first, then trial, error, and a dash of chemistry memory.
Happy drawing! And may your atoms always find their rightful connections.
How can I tell which atom is the central atom in Lewis structures like HCO2⁻ and HCO3⁻?
Identify the atom with the lowest electronegativity, often carbon in these ions. Carbon typically serves as the central atom, bonding to oxygen and hydrogen. Checking octet completion helps confirm this arrangement.
What role does the octet rule play in deciding atom connections in Lewis structures?
The octet rule helps exclude structures where atoms lack a full octet. For example, oxygen usually has an oxidation state of -2, so placing hydrogen on oxygen rather than carbon is guided by octet satisfaction and charge balance.
Why is it helpful to try drawing alternative Lewis structures with hydrogen bonded to different atoms?
Trying different hydrogen placements lets you see which structures are reasonable. If one alternative breaks octet rules or leads to unlikely oxidation states, you can discard it, revealing the most probable connectivity.
How does knowing common ion structures assist in identifying atom connectivity?
Familiarity with standard ions like bicarbonate means you recognize typical bonding patterns immediately. This avoids guesswork and confirms which atoms attach directly to the central atom based on well-studied examples.
Can understanding constitutional isomers clarify connections in polyatomic ions?
Yes. Comparing isomers such as formate (HCO2⁻) and bicarbonate (HCO3⁻) highlights how hydrogen connects either to carbon or oxygen. Drawing these helps confirm the specific atom hydrogen bonds to and the overall molecular layout.




Leave a Comment