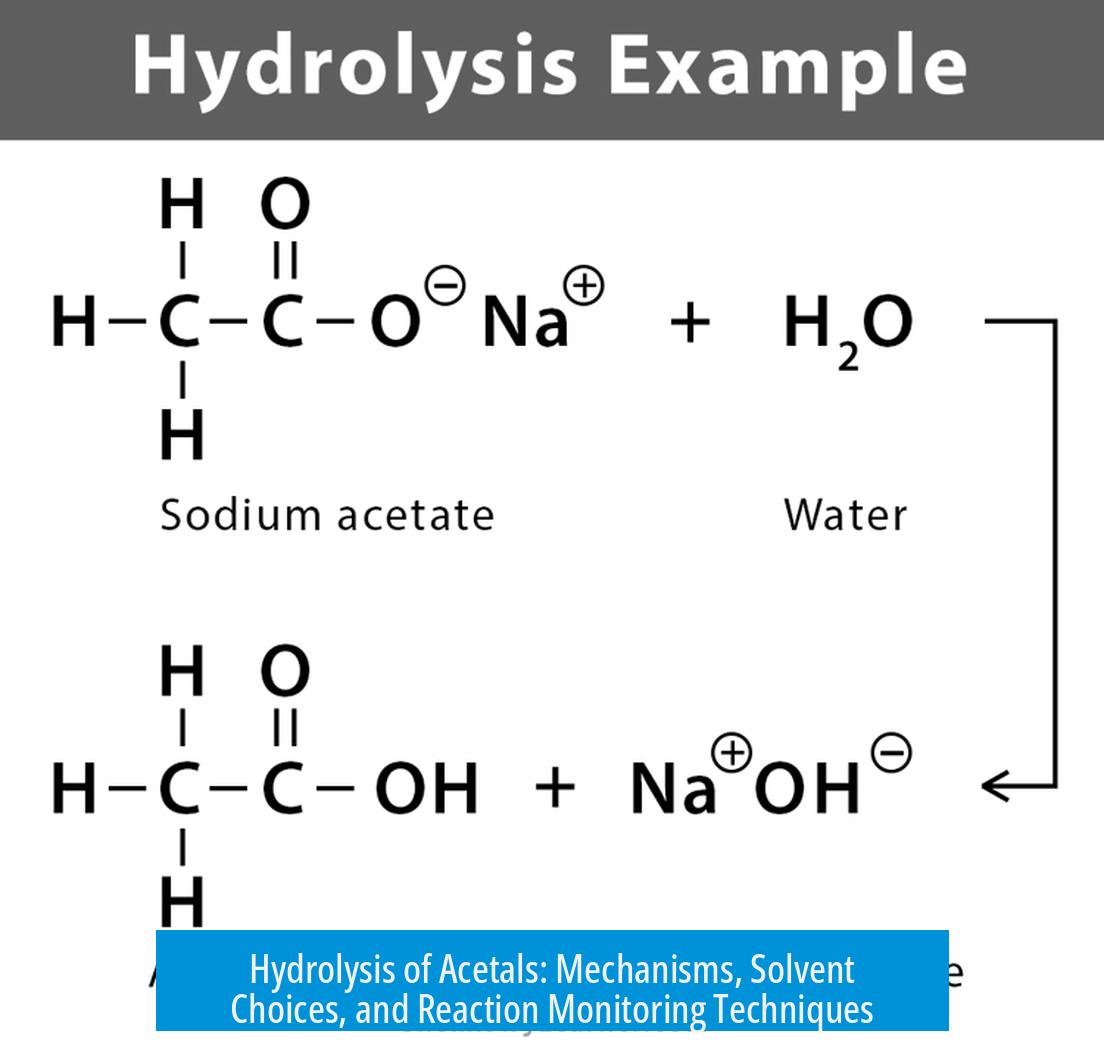
Hydrolysis of Acetals
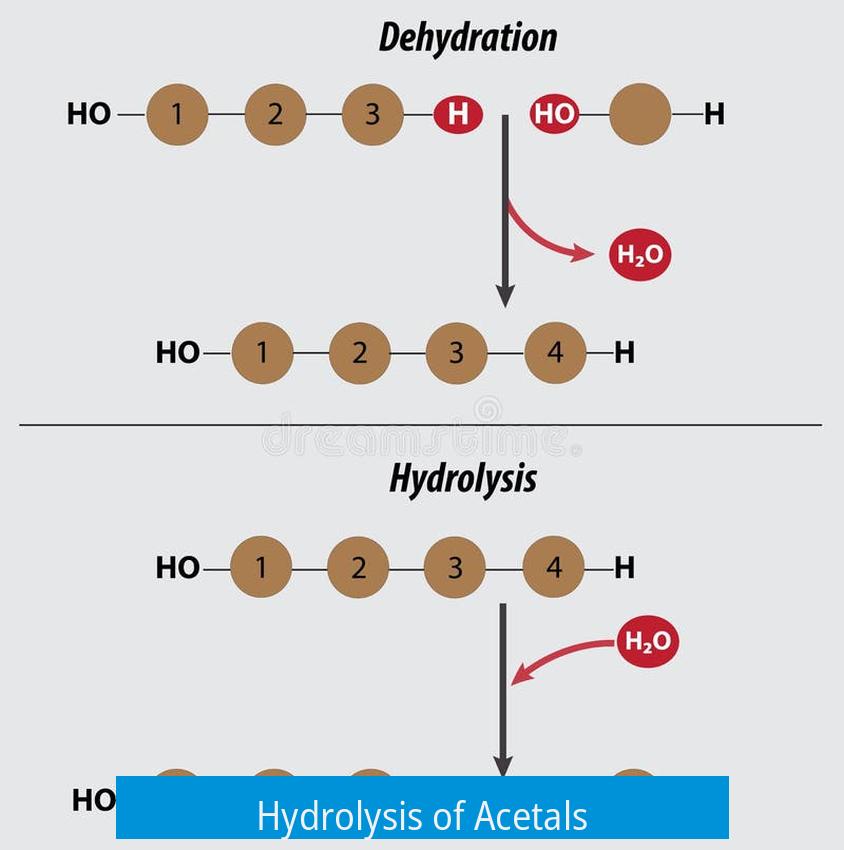
The hydrolysis of acetals occurs efficiently under acid-catalyzed conditions, converting acetals back into their carbonyl and alcohol components. This reaction typically requires mild acidic environments and controlled heating to prevent side reactions. Various acids and solvent choices influence the rate and selectivity of this transformation.
1. Acid-Catalyzed Deprotection Mechanisms
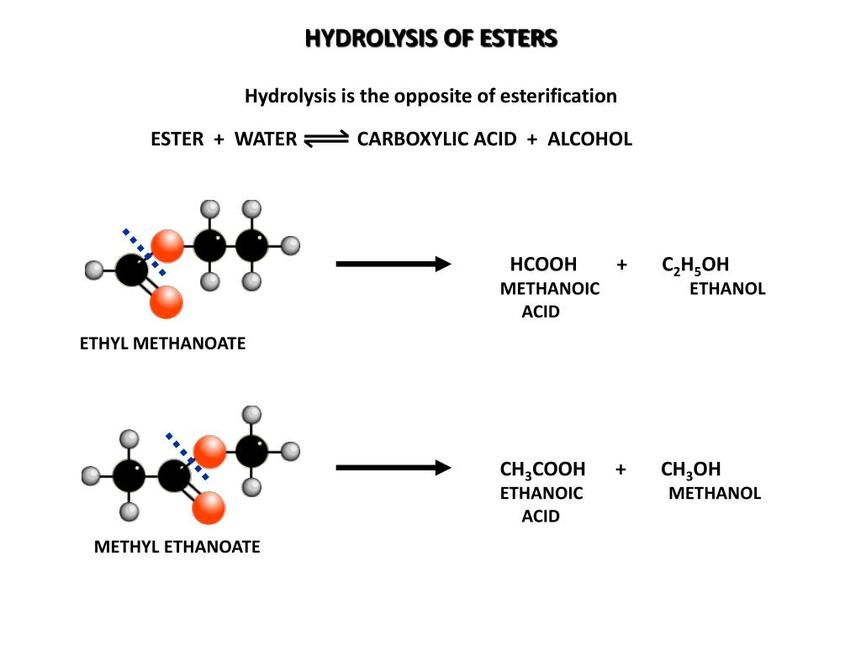
Acetals hydrolyze in the presence of acids through protonation, followed by cleavage of the acetal linkage. Commonly, refluxing acetals in acetic acid for 3–4 hours achieves effective deprotection. Alternatively, catalytic amounts of acid with gentle heating also facilitate hydrolysis.
- p-Tolylsulfonic acid acts as a robust catalyst in acetone with some water, promoting deprotection while maintaining mild conditions.
- Mineral acids like dilute hydrochloric acid (around 1M) in aqueous or acetic acid solutions provide an efficient yet mild environment avoiding excessive oxidation.
2. Solvent Effects and Work-Up
Acetal solubility can limit hydrolysis efficiency. Incorporating aprotic, water-miscible solvents such as tetrahydrofuran (THF) or 1,4-dioxane improves substrate dissolution and acid compatibility, including with HCl. However, these solvent mixtures may complicate post-reaction work-up steps, requiring careful separation and purification.
3. Avoiding Side Reactions
Strong acids like sulfuric acid may cause dehydration of glycols and potential oxidation of aldehydes formed during hydrolysis, affecting product integrity. Mild conditions prevent these issues and ensure selective acetal cleavage without further degradation.
4. Monitoring and Driving the Reaction
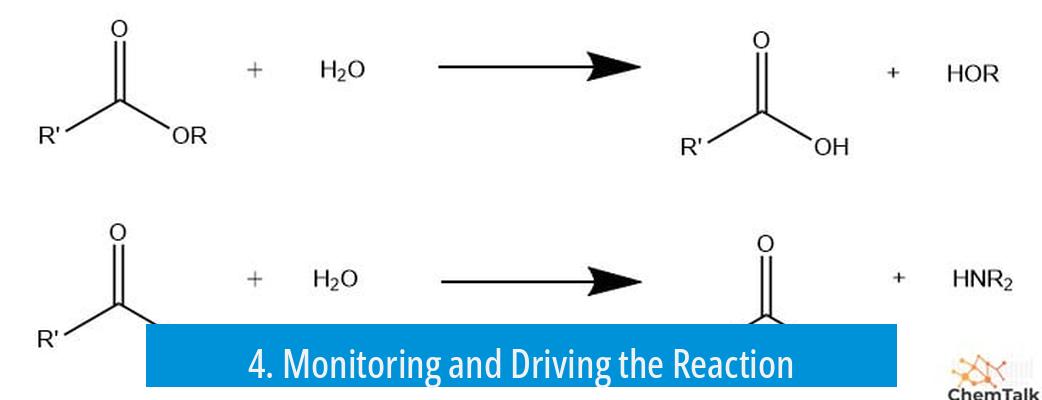
TLC can track acetal hydrolysis progress but necessitates special stains due to the reactivity and polarity changes of species involved.
Using a sacrificial aldehyde drives the equilibrium toward the desired product by transacetalization, exchanging the acetal moiety and pushing hydrolysis forward under acidic catalysis.
5. Additional Considerations
- Oxidative work-ups post-hydrolysis, employing reagents like permanganate or periodate, can facilitate downstream transformations such as hydrazone formation.
- Understanding the specific experimental goal behind hydrolysis aids in optimizing conditions and selecting reagents.
Key Takeaways
- Acid-catalyzed hydrolysis under mild heating effectively cleaves acetals to carbonyls.
- Mild acids (e.g., dilute HCl, p-tolylsulfonic acid) minimize side reactions.
- Aprotic, water-miscible solvents improve acetal solubility but may complicate work-up.
- Monitoring requires specialized TLC stains due to polarity shifts.
- Inclusion of sacrificial aldehydes can promote reaction completion by transacetalization.
- Strong mineral acids risk dehydration and oxidation, generally avoided for sensitive substrates.
- Oxidative reagents post-hydrolysis enable further functional transformations.
Unlocking the Secrets of the Hydrolysis of Acetals: A Practical Guide
Wondering how to break down those stubborn acetals? The hydrolysis of acetals is primarily achieved under acid catalysis, often by refluxing in acetic acid or using catalytic amounts of acids like p-tolylsulfonic acid or HCl in aqueous or mixed solvents. Simple enough? Grab a stir bar and let’s dive deeper into what makes this reaction tick and how best to master it.
Acetals are popular protective groups in organic synthesis. But when the time comes to remove them, hydrolysis is your friend. The catch? It’s a delicate dance of acids, solvents, and conditions.
Acid-Catalyzed Deprotection: The Classic Workhorse Approach
The most straightforward way is acid-catalyzed hydrolysis. Ever refluxed an acetal in acetic acid for 3-4 hours? That’s classic territory. The heat and acid cooperate to break the durable acetal back into its aldehyde or ketone and the corresponding diol. For many chemists, this method is their first port of call—effective and predictable.
If acetic acid sounds a bit too “old-school”, consider p-tolylsulfonic acid (p-TsOH). It’s a sulfonic acid that’s just a bit gentler than sulfuric acid but still packs a punch. Dissolve it in acetone with a splash of water, and you create a friendly environment for your acetal to unwind. This solvent mix improves solubility without turning the mixture into a chemical soup. Gentle heating with catalytic acid like p-TsOH is often enough to coax the acetal off its perch without overdoing it. Pro tip: It’s worth checking out resources like the organic-chemistry.org protective groups guide for detailed protocols.
Choosing Your Solvent: The Unsung Hero of Hydrolysis
Getting acetals to cooperate often depends on solvent choice. Since acetals sometimes don’t dissolve well in simple aqueous acid, adding an aprotic and water-miscible solvent can do wonders. Tetrahydrofuran (THF) or 1,4-dioxane are prime candidates here. Their compatibility with acids such as HCl lets you fine-tune your reaction environment. They increase solubility, meaning your reactants meet each other faster and react cleaner.
But here’s the kicker—work-up with these solvents can get annoying. Expect a bit of extra effort in purification since these solvents don’t just evaporate like water or acetic acid. Your crude mixture might turn into a soap opera of emulsions and sticky residues. Worth it? Usually, yes, for better reaction control and yield.
Strong Mineral Acids: Handle with Care
Strong acids like sulfuric acid might appear tempting due to their high acidity. However, they can bring drama, not just chemistry. Sulfuric acid can dehydrate the glycol byproduct and even oxidize the freshly freed aldehyde, spoiling your hard work. Mild but effective conditions, such as 1M HCl in water or acetic acid, offer a safer alternative without turning your reaction into a serial side-reaction saga.
Watching the Cure: TLC Monitoring with a Twist
How do you track if your acetal has bowed out gracefully? Thin-layer chromatography (TLC) is the trusty spy. Be prepared, though—you’ll need special staining since acetals might not show up clearly with standard UV detection. This means extra attention but gives you the confidence to call the reaction complete without guessing.
Giving the Reaction a Nudge: Driving Forces and Smart Strategies
Hydrolysis is reversible; sometimes you need a little extra push. Enter the sacrificial aldehyde—a subtle game changer. By introducing a sacrificial aldehyde, you encourage transacetalization, effectively shifting the equilibrium towards your desired aldehyde product. It’s like tempting the system with a better deal, and it takes the bait happily.
It’s a clever strategy when you want to make sure the hydrolysis doesn’t stall midway. For acid-catalyzed reactions, this trick is especially handy, smoothing the road to completion.
Cleaning Up: Oxidative Work-Up for the Hangover
If you need to tidy up after hydrolysis or eliminate stubborn byproducts, oxidative agents like permanganate or periodate can help. For example, after hydrolysis, periodate can cut through glycol remnants, sometimes followed by forming hydrazones to capture aldehydes clearly. Think of it as a cleanup crew ensuring your reaction site looks pristine.
Your Personal Reaction: What Are You Trying to Achieve?
One final question—why are you hydrolyzing that acetal? Whether it’s to unmask a reactive aldehyde for further synthesis, prepare a substrate for coupling, or just to test a protective strategy, understanding your goal frames your method choice. Gentle conditions preserve sensitive molecules, whereas vigorous ones might be necessary for stubborn acetals.
Expert Takeaway
Hydrolyzing acetals isn’t one-size-fits-all. Best results blend thoughtful acid choice, solvent selection, and reaction conditions tailored to your substrate’s personality. Reflux in acetic acid or catalytic p-TsOH with water works well for many. For tricky waters, mixing in THF or 1,4-dioxane helps. Avoid harsh sulfuric acid when oxidation risk looms.
Keeping a watchful eye with TLC and possibly using sacrificial aldehydes sharpens your success odds. Add oxidative work-ups if cleanup is part of your journey. As always, start mild and adjust. Hydrolysis of acetals is both chemistry and art.
Bonus Trivia
Did you know acetals often mask aldehydes to protect them from oxidation during complex steps? Their stability under neutral or basic conditions is a boon, making them switchable tools rather than permanent fixtures in a synthesis. So the hydrolysis stage is the grand finale where the disguise drops!
Feel ready to tackle your hydrolysis now? What’s your favorite acid or solvent combo to get those acetals to unwind? Share your stories and triumphs—because every lab hero deserves credit.
Q1: What acids are commonly used for hydrolyzing acetals?
Acetic acid at reflux, p-tolylsulfonic acid in acetone/water, and mild mineral acids like 1M HCl are commonly used. Catalytic amounts of acid with gentle heating also work well.
Q2: How can I improve acetal solubility during hydrolysis?
Adding aprotic, water-miscible solvents such as THF or 1,4-dioxane helps improve solubility while being compatible with acids like HCl.
Q3: Why should sulfuric acid be avoided for acetal hydrolysis?
Sulfuric acid can cause dehydration of the glycol and possible oxidation of the aldehyde, leading to unwanted side reactions.
Q4: How can I monitor the progress of acetal hydrolysis?
TLC is useful for monitoring but requires a special stain to visualize the reaction progress effectively.
Q5: What strategies can drive the hydrolysis reaction toward the desired product?
Using a sacrificial aldehyde to transacetalize can shift the equilibrium and drive the hydrolysis toward completion under acidic conditions.



Leave a Comment