Aromatic, Nonaromatic, and Neither Explained
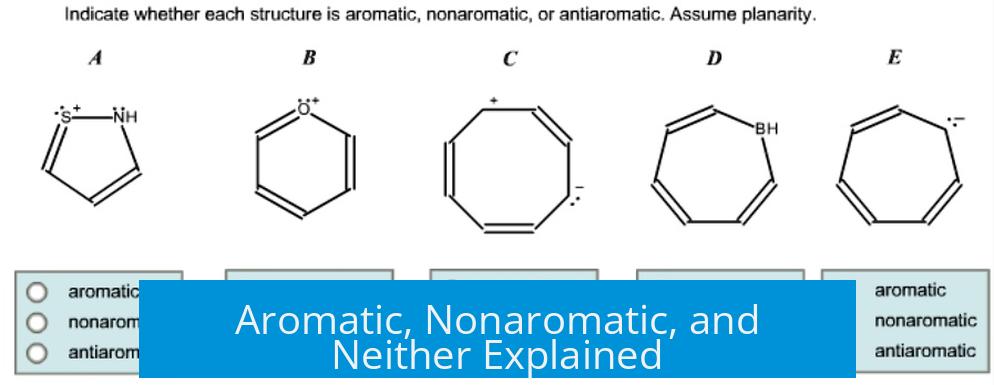
Aromaticity describes a molecule’s special stability arising from a planar, cyclic, fully conjugated system with (4n + 2) π electrons, known as Hückel’s rule. Nonaromatic molecules lack this strict conjugation or planarity, while neither applies to structures that do not fit aromatic or anti-aromatic criteria.
Understanding Conjugation and Resonance
Conjugation, resonance, and mesomeric structures refer to the delocalization of electrons within molecules. This delocalization allows multiple resonance forms, sharing electron density over adjacent p-orbitals. These terms describe general electron mobility but do not guarantee aromaticity.
What Makes a Molecule Aromatic?
- Planarity: The molecule is flat so p-orbitals can overlap.
- Cyclic Structure: Electrons must be delocalized in a ring.
- Full Conjugation: Continuous overlap of p-orbitals around the ring.
- Electron Count: The system contains (4n + 2) π electrons, where n is an integer (0, 1, 2, …).
These rules define special stability. Aromatic molecules exhibit unique chemical behavior and lower reactivity compared to nonaromatic counterparts.
Anti-Aromaticity and Destabilization
Anti-aromatic compounds meet planarity and conjugation but have 4n π electrons. This configuration destabilizes the molecule due to electron-electron repulsion in the delocalized system. Anti-aromatic compounds are rare and highly reactive.
Nonaromatic and Neither
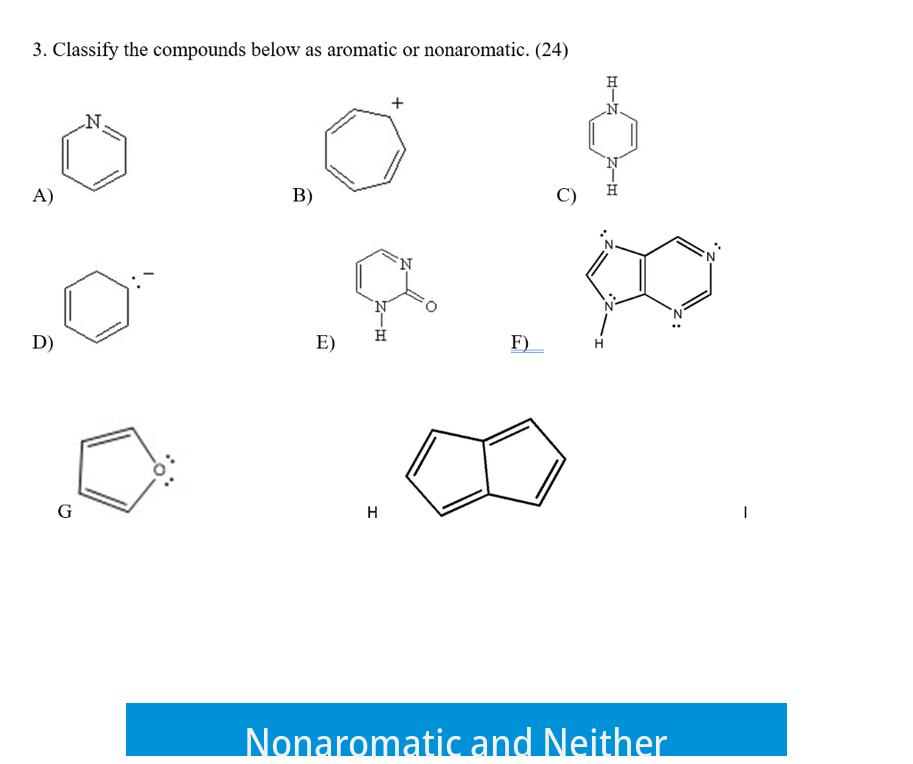
Nonaromatic molecules do not fulfill the conjugation or planarity needed for aromaticity but do not destabilize like anti-aromatic molecules. They may have isolated double bonds or lack continuous overlap of p-orbitals.
The category “neither” applies when a molecule does not meet criteria for aromatic, anti-aromatic, or fully nonaromatic systems. For example, non-planar cyclic compounds often fall here.
Differentiating General Conjugation from Aromaticity
Conjugation alone, meaning electron delocalization, is common in many molecules. Aromaticity is a subset of this concept with strict structural and electronic requirements. Mixing these terms leads to confusion.
Summary of Key Points
- Aromaticity requires planar, cyclic, fully conjugated systems with (4n + 2) π electrons (Hückel’s rule).
- Anti-aromatic molecules are planar and conjugated but have 4n π electrons, causing destabilization.
- Nonaromatic molecules lack full conjugation or planarity; they neither stabilize nor destabilize significantly via delocalization.
- Conjugation, resonance, and mesomeric effects describe electron delocalization broadly; aromaticity is a special condition within this.
- Distinguishing aromaticity from general conjugation avoids misunderstandings in molecular stability and reactivity.





Leave a Comment