Does Heating Drive NaBH4 Reactions to Completion?
Heating sodium borohydride (NaBH4) reactions is generally not used to drive them to completion because heating rapidly decomposes NaBH4, leading to uncontrolled exothermic reactions and hydrogen gas release rather than clean reduction.
How Heating Affects NaBH4 Stability

NaBH4 decomposes quickly when heated, especially in alcoholic solvents. This decomposition generates hydrogen gas and causes an exothermic runaway reaction. Such behavior complicates control over reaction progress and safety.
Reduction Reactions Involving NaBH4
In some cases, the hydrogen produced by NaBH4 decomposition at elevated temperatures might reduce functional groups like imines. However, this indirect reduction is less predictable and could be due to decomposition, not direct hydride transfer.
Typical Conditions for NaBH4 Reductions
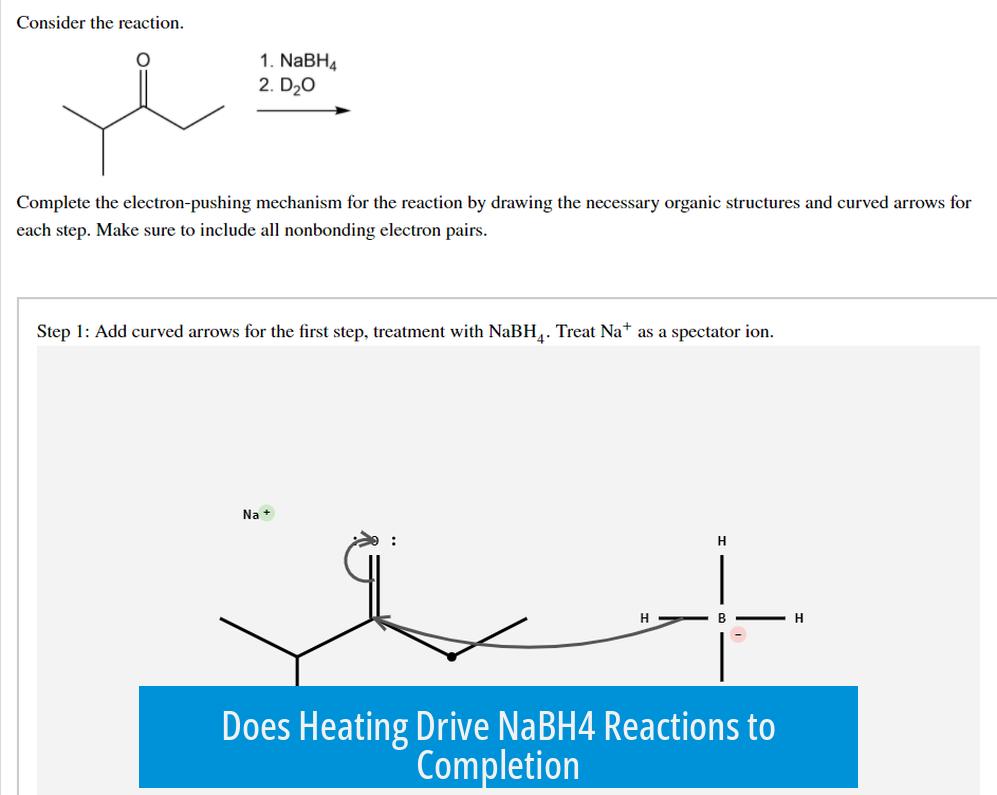
- Reactions with NaBH4 often proceed at room temperature or below.
- Catalyzed reductions (e.g., nitro group reductions) commonly use metals like NiCl2 at low temperatures (around 0°C).
- Extended reaction times rather than heating usually allow the reaction to reach completion.
Monitoring Reaction Progress
Analytical techniques such as thin-layer chromatography (TLC) with specific stains (e.g., ninhydrin for amines) verify whether the reduction is complete or if decomposition products dominate.
| Aspect | Effect of Heating NaBH4 |
|---|---|
| Stability | Rapid decomposition |
| Gas Formation | Hydrogen gas released |
| Reaction Control | Runaway exothermic reaction |
| Reduction Efficiency | Indirect, less predictable |
| Preferred Conditions | Room temperature or cooling |
Summary of Key Points
- Heating NaBH4 triggers hazardous decomposition and hydrogen evolution.
- Reduction often completes over time without heating.
- Hydrogen generated by decomposition can reduce some substrates but unpredictably.
- Use low-temperature conditions and metal catalysts for nitro reductions with NaBH4.
- Monitor reactions by TLC or other analytical methods to confirm completion.
Can heating drive NaBH4 reactions to completion?
No. Heating causes NaBH4 to rapidly decompose, not complete the reaction. It generates hydrogen gas and can lead to uncontrolled exothermic reactions, especially in alcoholic solvents.
Does NaBH4 release hydrogen gas when heated?
Yes. Heating NaBH4 decomposes it, releasing hydrogen gas. This hydrogen can sometimes reduce compounds like imines, but the reaction is unstable and unsafe.
Is heating necessary for nitro group reduction with NaBH4?
No. Nitro reductions with NaBH4 usually require a catalyst like NiCl2 and are done at low temperatures, not by heating. Heating can cause decomposition rather than reduction.
How can I confirm if a NaBH4 reaction is complete or if decomposition occurred?
Use TLC with ninhydrin stain to check for amines. This helps distinguish between true reaction products and those formed by decomposition after heating.



Leave a Comment