Assessment of Leaving Groups in Organic Reactions

Leaving group quality depends largely on the reaction context and matched reactivity rather than an absolute good or bad classification. The leaving group’s ability varies with the type of reaction, reaction conditions, and the nucleophile involved.
Leaving Groups in Acyl Substitution
In acyl substitution reactions like ester hydrolysis, alkoxide (RO-) groups serve as leaving groups. Although alkoxides are generally considered poor leaving groups due to their strong basicity, under basic conditions, they can leave effectively.
- Strong nucleophiles such as Grignard reagents (R-MgBr) and LiAlH4 can displace alkoxide groups because these nucleophiles form even less favorable leaving groups themselves.
- For example, in the nucleophilic addition of a Grignard reagent to an ester, the intermediate alkoxide anion can expel either an alkyl or an alkoxide ion; here, alkoxide is favored as it is a better leaving group than the carbanion.
Leaving Groups in Nucleophilic Aromatic Substitution (SNAr)
Alkoxide ions are uncommon as leaving groups in SNAr reactions. Standard leaving groups include diazonium, fluoride, chloride, and nitroso groups.
This differentiation illustrates that the nature of the aromatic system and the leaving group’s stability in the given reaction mechanism play crucial roles.
Halogen Leaving Groups
| Halogen | Leaving Group Quality | Typical Behavior |
|---|---|---|
| Iodide (I-) | Sometimes good | Useful but prone to side reactions; selective use advised |
| Fluoride (F-) | Often poor | Usually inert under many conditions; rarely leaves |
Oxygen-Based Leaving Groups
The leaving ability of oxygen-based groups depends significantly on substituents attached to oxygen. Modifications can enhance or reduce leaving tendencies.
This flexibility allows tailoring leaving group quality to suit specific reaction conditions.
Key Takeaways
- Leaving group quality depends on reaction context and matched reactivity.
- Alkoxide groups leave effectively in acyl substitutions under basic conditions.
- Alkoxides are poor leaving groups in SNAr reactions; halogens and diazoniums are preferred.
- Iodide can be effective but may cause side reactions; fluoride is typically inert.
- Substituents on oxygen affect the leaving group ability of oxygen-based groups.
Assessment of Leaving Group: What Makes a Leaving Group Shine—or Flop?
Wondering how to assess a leaving group in organic reactions? Well, it’s less about a simple “good” or “bad” label and more about understanding the chemistry context. The real secret? Matched reactivity.
So, here’s the lowdown: leaving groups don’t operate in a vacuum. Their success depends on the reaction type, the conditions, and how well they “get along” with the nucleophile and substrate. Let’s dive into some real-world examples and surprising twists you might not have expected.
Acyl Substitution: When Alkoxide Steps Off the Stage (Sometimes)
Start here: ester hydrolysis, a classic acyl substitution. You know, when an ester meets a nucleophile and kicks off its alkoxide group (–OR). Is alkoxide a good leaving group? Usually, no.
But hold on! Under basic conditions, alkoxide can leave the party because free RO– ions already roam the media. That’s key. When there’s already plenty of RO– hanging around, it’s less shocking if another alkoxide bugs out.
- Imagine you’re adding an alkyl group to an ester with a Grignard reagent (R-MgBr). The nucleophile hits, and the alkoxide anion forms.
- This alkoxide then shoves out either R– or RO–, picking the lesser evil as it exits. Turns out, alkoxide is a much better leaving group than a carbanion. Fancy that!
Even stronger nucleophiles like lithium aluminum hydride (LiAlH4) can displace alkoxide, making the reaction thermodynamically favored. So, while alkoxide isn’t the flashiest leaving group, it plays its part perfectly when conditions match.
SNAr Reactions: Alkoxide? Definitely Not on the Guest List

Switch gears to nucleophilic aromatic substitution (SNAr). Alkoxide leaving groups here? Near impossible at the undergraduate level.
Why? Because the aromatic system is picky. Leaving groups like diazoniums, fluorides, chlorides, and nitros fit the role well. Alkoxides just don’t cut it; they’re too stubborn to leave the ring.
Consider it like a party invitation—only the fanciest halogens or diazonium ions get an official pass to leave the aromatic system.
Halogen Leaving Groups: The Good, the Bad, and the Side-Reaction-Prone
When assessing halogen leaving groups, the story is fascinating and nuanced.
- Iodide: Fantastic at leaving when you want, but watch out! It’s prone to side reactions that can crash your reaction party.
- Fluoride: Usually inert and generally terrible as a leaving group in most organic reactions. Fluoride doesn’t like to break free.
The takeaway? Don’t just slap a “good” or “bad” label on halogens. It depends on your synthetic goal and reaction design.
Oxygen-Based Leaving Groups: You’ve Got Influence on Reactivity
Oxygen atoms hosting the leaving group? Their leaving ability hugely depends on what’s attached to the oxygen.
Different substituents on oxygen modulate the reactivity. Think of it like a VIP pass for leaving groups—sometimes you can change the entourage (substituents) and improve your oxygen leaving group’s exit strategy.
Matched Reactivity Beats the Good/Bad Binary
The true art of assessing leaving groups lies in “matched reactivity.”
Rather than defaulting to “this group is good” or “this one is bad,” consider the entire reaction environment and participants:
- Who’s attacking? (nucleophile)
- What’s the medium? (acidic or basic conditions)
- What does the substrate look like?
For example, –OR leaving groups might seem weak anywhere else, but in basic acyl substitution where free RO– exists, they behave perfectly. Grignard reagents know how to push them out, scoring big thermodynamic wins.
So, matched reactivity isn’t just chemistry jargon—it’s the practical, real-world way to predict results.
Summary Table of Common Leaving Groups and Their Context
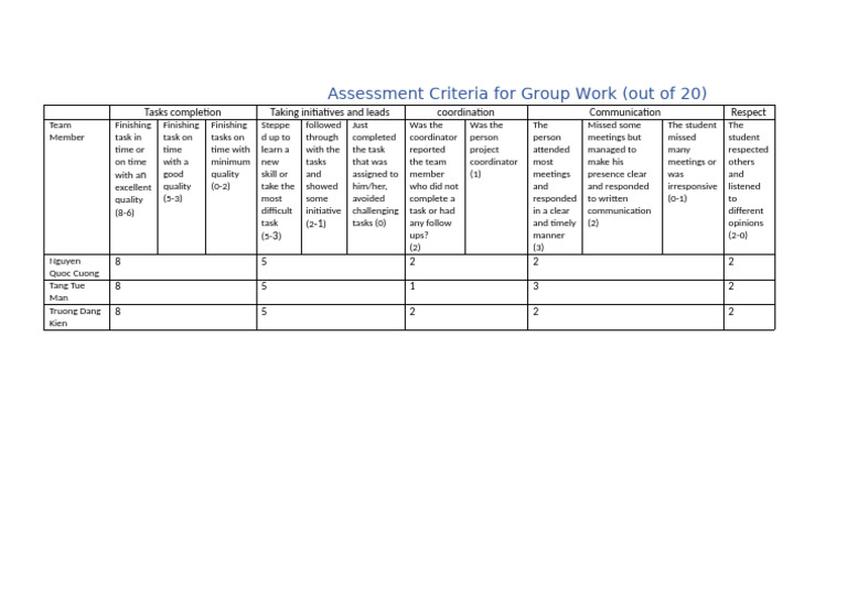
| Leaving Group | Typical Reaction Context | Leaving Group Quality | Notes |
|---|---|---|---|
| Alkoxide (–OR) | Acyl substitution (ester hydrolysis, Grignard addition) | Conditional good | Good when free RO– present; displaced by strong nucleophiles |
| Iodide (I–) | Various SN1/SN2 | Good | Prone to side reactions; good leaving group but use cautiously |
| Fluoride (F–) | SNAr and others | Poor | Usually inert, poor leaving group |
| Diazonium (N2+) | SNAr | Excellent | Highly reactive, excellent in aromatic substitution |
| Oxygen-based (depends on substituents) | Varied | Variable | Reactivity modulated by attached groups |
Why Should You Care About Leaving Groups?
Understanding leaving groups does more than get you through organic exams—it helps you design smarter syntheses, predict reaction outcomes, avoid failures, and even invent new transformations.
For instance, knowing that alkoxide isn’t great generally—but perfect in basic acyl substitution—can save you hours of frustration. Likewise, appreciating halogens’ quirks allows for smarter reagent choices.
So next time you see a reaction step, ask: “Who’s leaving, and why?” Because sometimes the star isn’t the one entering but the one exiting.
Final Thought
Assessing leaving groups isn’t black or white. It’s a **nuanced dance** of chemistry partners. Mastering this helps in both academic learning and lab success. What’s your experience with tricky leaving groups? Ever been surprised by a “bad” group behaving brilliantly?
Drop your stories or questions below—let’s bring leaving groups out of the shadows!





Leave a Comment