Which Anti-Conformation Is More Stable for Two Sets of Conformational Isomers?
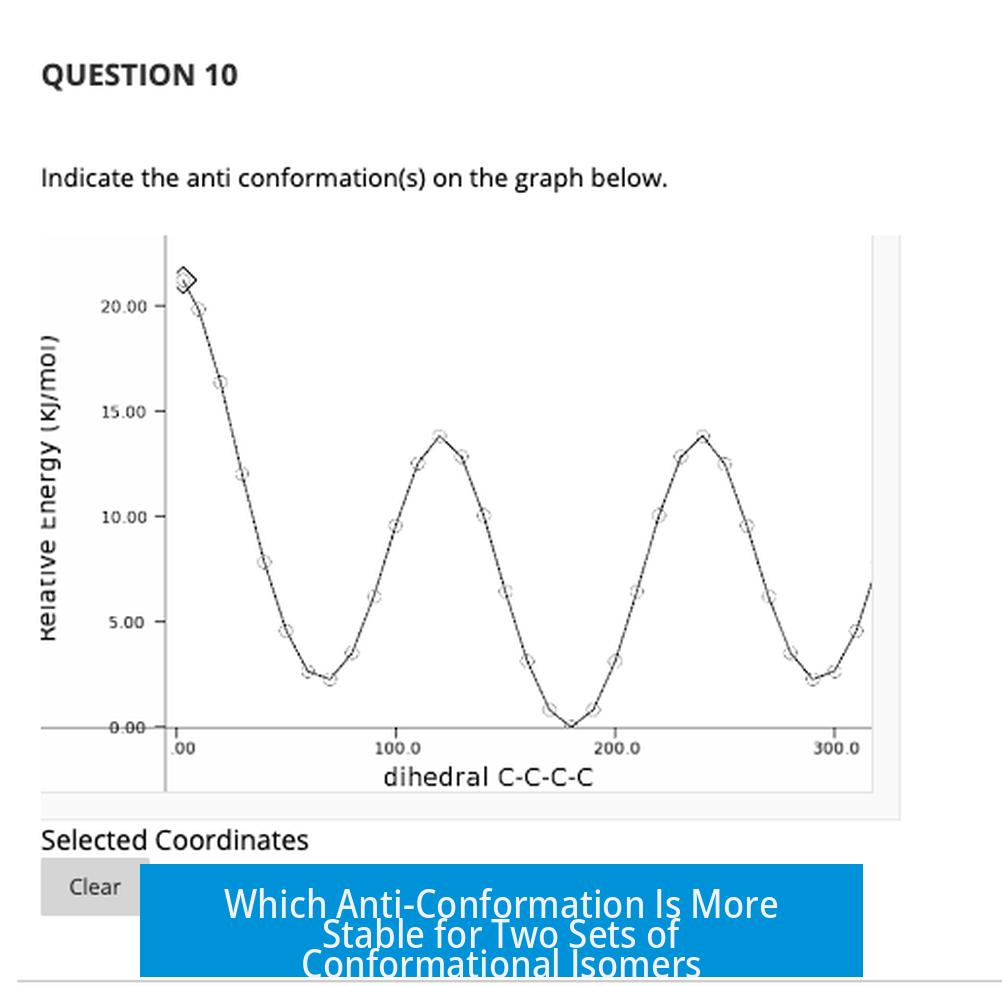
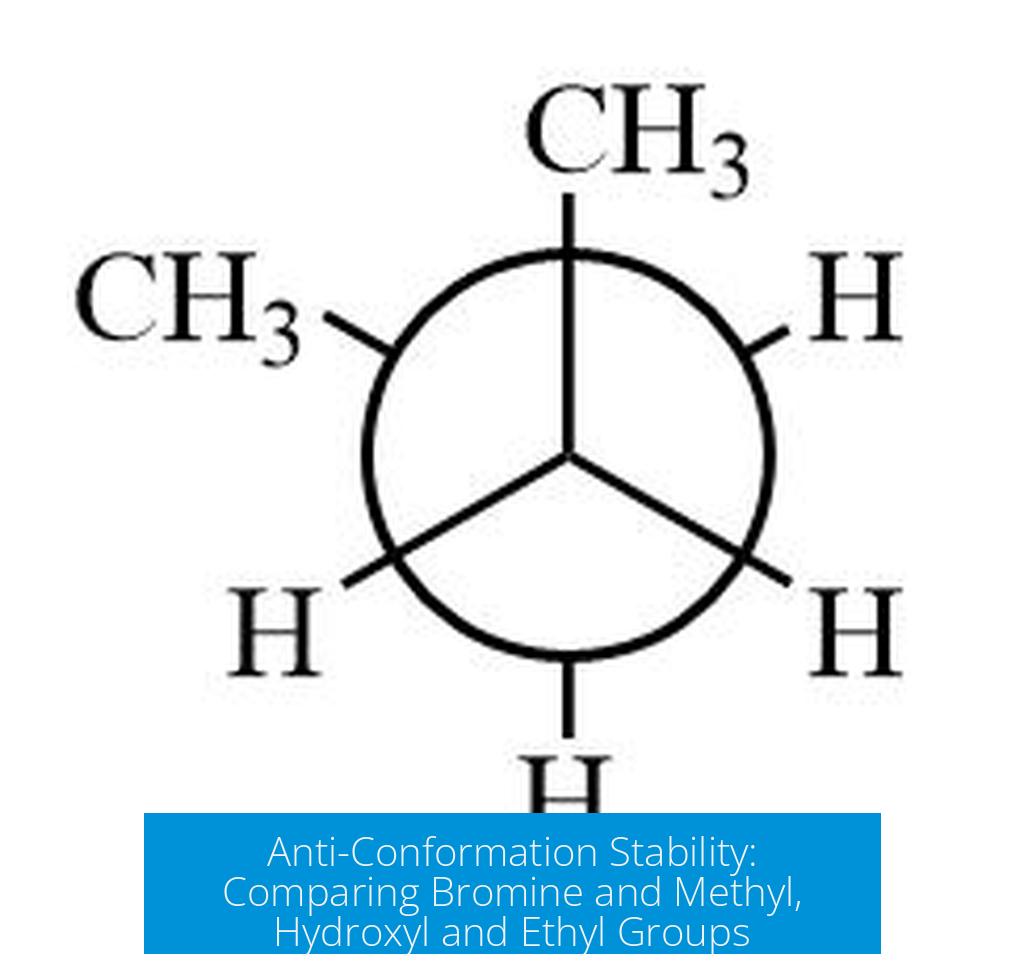
The anti-conformation that is more stable depends on the size of substituents and the potential for favorable interactions like hydrogen bonding. For the two sets discussed, the top left conformer with bromine is more stable than the top right with a methyl group due to less steric hindrance. The bottom left conformer is more stable than the bottom right because of hydrogen bonding between hydroxyl groups and ethyl groups being anti to each other.
Stability in the First Set: Bromine vs Methyl
- The top left conformer has bromine opposite to a bulky group, while the top right has a methyl group in place of bromine.
- Bromine is smaller in atomic size compared to the methyl substituent, creating less steric crowding.
- This size difference means fewer spatial clashes and less torsional strain.
- As a result, the top left conformer, with the bromine substituent, holds lower energy and is more stable.
Stability in the Second Set: Hydroxyl and Ethyl Groups

- The bottom left conformer allows hydrogen bonding between the hydroxyl (OH) groups.
- Hydrogen bonds stabilize the molecule by lowering its overall potential energy.
- In addition, the ethyl groups in this conformer are positioned anti to one another.
- This anti arrangement minimizes steric hindrance between large alkyl groups.
- Consequently, the bottom left conformer is more stable than the bottom right variant.
Key Factors Affecting Anti-Conformation Stability
| Factor | Effect on Stability |
|---|---|
| Substituent Size (Br vs CH3) | Smaller substituents reduce steric strain, increase stability. |
| Hydrogen Bonding (OH groups) | Favorably lowers molecule’s energy; stabilizes conformation. |
| Anti Positioning of Alkyl Groups (ethyl) | Minimizes steric clashes, enhances conformer stability. |
Summary of Stability Trends
- Smaller atoms or groups create lower steric hindrance, favoring stability.
- Intramolecular hydrogen bonding strongly stabilizes conformers.
- Anti orientation of bulky groups reduces strain and increases stability.
Which anti-conformation is more stable between the top left and top right conformers?
The top left conformer is more stable because bromine is smaller than a methyl group. This reduces steric hindrance, making the top left conformer favored.
Why does the bottom left conformer have greater stability than the bottom right?
The bottom left conformer benefits from hydrogen bonding between hydroxyl groups. Also, ethyl groups are anti to each other, minimizing steric clashes.
How does the size of substituents affect anti-conformation stability?
Smaller substituents, like bromine compared to methyl, decrease steric hindrance. Less steric strain means more stable anti-conformations.
What role does hydrogen bonding play in anti-conformation preference?
Hydrogen bonding between OH groups stabilizes conformers by providing intra-molecular attractions. This effect favors conformers where such bonding is possible.
How do the arrangements of ethyl groups influence stability?
When ethyl groups are placed anti to each other, steric interactions reduce. This arrangement leads to greater stability in the anti-conformation.


Leave a Comment