Luciferase/Luciferin In Vitro Bioluminescence Demonstration

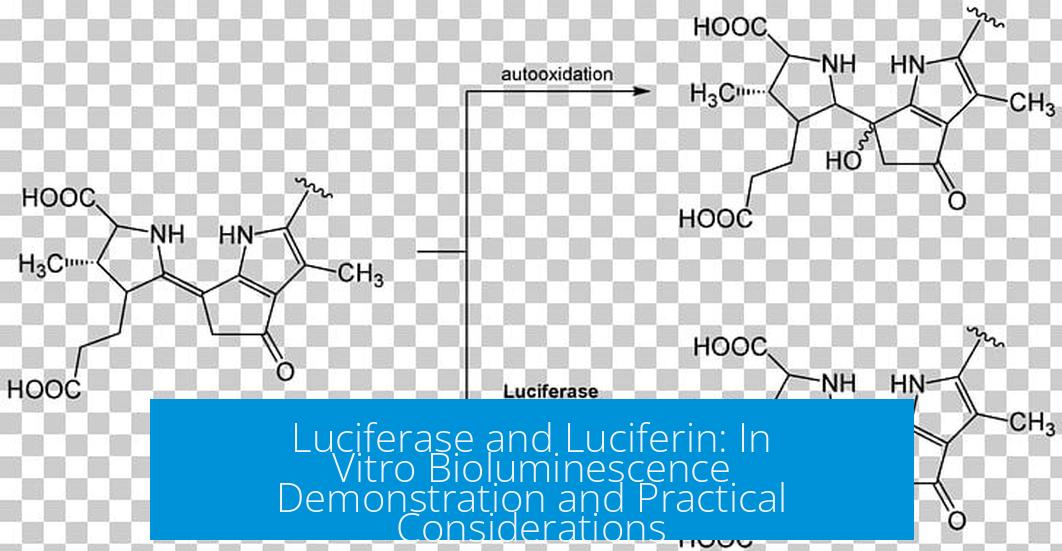
Luciferase and luciferin together enable bioluminescence, which can be clearly demonstrated in vitro. The visibility of light depends on the luciferase type, concentrations, and reaction conditions. Specifically, firefly luciferase with adequate substrate and cofactors produces light visible even at ambient light, while other luciferases often require darkroom conditions for detection.
Types of Luciferase Used in Bioluminescence
Several luciferase enzymes facilitate bioluminescence. The most common include:
- Firefly (beetle) luciferase: The classical and widely used enzyme with intense, easily detectable light output.
- Renilla luciferase: Derived from sea pansy, emits blue-green light but usually requires sensitive detection equipment.
- Gaussia luciferase: A smaller enzyme with high light output but shorter signal duration.
- Nano luciferase: Engineered for high brightness and stability, used in research but requires special substrates.
The luciferase variant selection depends on application requirements, including signal color, stability, and brightness.
Visual Detection of Luminescence
Most luciferase assays do not produce visible light under ambient laboratory lighting conditions. For example, the glow of Renilla or Gaussia luciferase is only detectable with photodetectors or imaging cameras in darkness.
In contrast, horseradish peroxidase (HRP) used in western blot detection paired with chemiluminescent substrates can produce visible light under darkroom conditions. This sets a comparative benchmark, demonstrating that visible luminescence often requires controlled environments.
Firefly Luciferase: Brightness and Concentration for Visibility
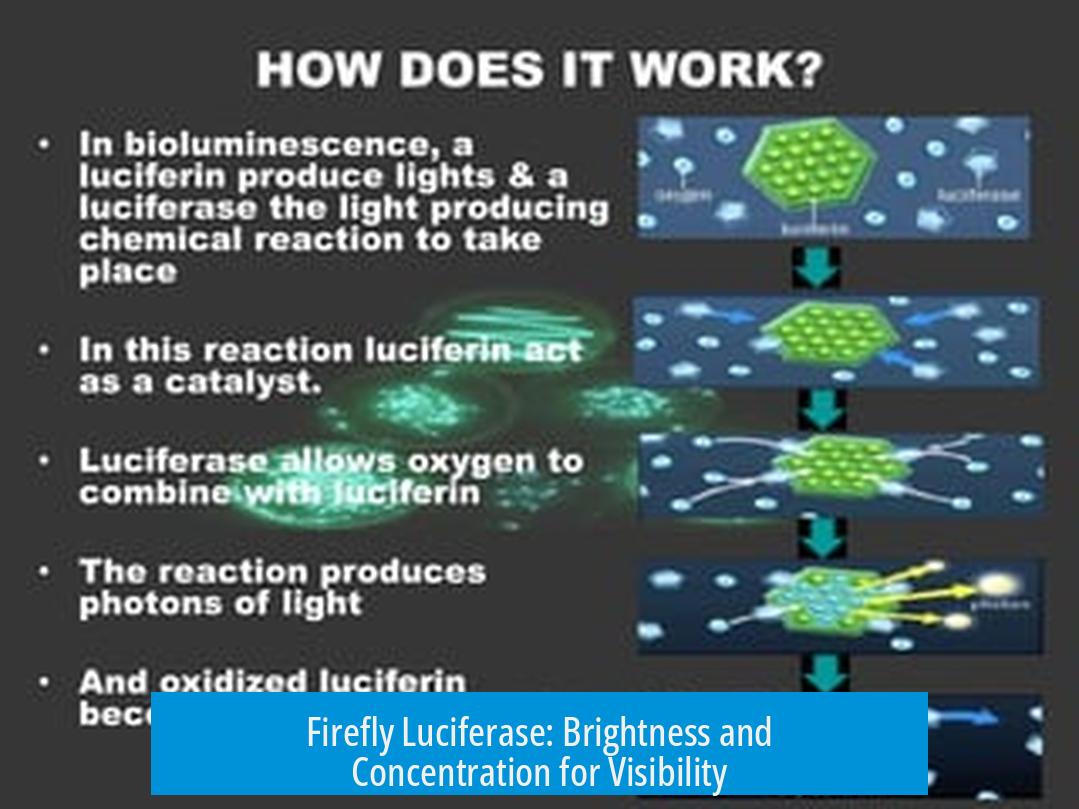
Firefly luciferase is distinctive because it can emit visible light under ambient conditions if used at sufficient concentrations. Key parameters for in vitro detection include:
| Component | Typical Concentration | Function/Effect |
|---|---|---|
| Luciferin (LH2) | 0.5 mM | Substrate; necessary for light emission |
| ATP | 1 mM | Cofactor; provides energy for the reaction |
| Firefly luciferase | 0.03 – 0.1 mg/mL | Enzyme; concentration correlates with brightness |
At this concentration, the light emitted is bright enough to be seen without specialized equipment and even in ambient light. When luciferase is diluted to 0.001–0.01 mg/mL, it still produces visible glow in the dark but less intense.
For reference, purified firefly luciferase expressed in E. coli enjoys use in in vitro assays. Reaction buffer preparation is crucial to maximizing visibility.
Color of Light Emission from Luciferase
Firefly luciferase typically emits green to yellow-green light. Some enzyme variants have been engineered or isolated that shift the emission toward red wavelengths. The color depends on the interaction between luciferase and luciferin, as well as environmental factors such as pH and temperature.
Other luciferases emit different colors, for instance:
- Renilla luciferase: Blue-green emission
- Gaussia luciferase: Blue emission
This spectral diversity enables multiplexing and various research applications.
Use of Live E. coli Producing Luciferase
In addition to purified enzymes, live bacteria engineered to produce firefly luciferase emit visible light when provided with luciferin. These glowing bacteria demonstrate bioluminescence in the form of luminous blobs or streaks visible in the dark.
This property finds creative use in visualizing bacterial patterns (“glowing bacterial art”) and functional studies. The brightness varies according to bacterial density, luciferase expression levels, and luciferin supply.
Reagents, Costs, and Preparation
Luciferin and ATP are the primary reagents for luciferase assays. Cost considerations are important for experimental design:
- Luciferin (Firefly): Approximately 1 g costs $250, enough for about 7 liters of 0.5 mM solution.
- ATP (Sigma): 1 g costs around $54, a common cofactor in biochemical assays.
These prices influence scale and frequency of experiments. Optimization to minimize reagent volumes is standard in research labs.
Optimizing Reaction Buffer Composition
Typical reaction conditions employ saturating concentrations of substrates:
- 0.5 mM luciferin
- 1 mM ATP
However, lower concentrations (0.1 mM luciferin and 0.1 mM ATP) can still support luminescence in many cases.
Adding protein stabilizers and cofactors can enhance brightness and extend signal duration:
- Bovine Serum Albumin (BSA): 1 mg improves brightness.
- Coenzyme A: Enhances signal strength and prolongs glow.
These additives are optional depending on the assay purpose. They contribute to maintaining luciferase stability during reaction.
Practical Aspects for Obtaining Luciferase
Commercial luciferase is often costly at analytical purity levels. For example, 1 mg can cost approximately $90. However, research laboratories commonly produce luciferase recombinantly in E. coli, significantly reducing expenses and increasing availability.
Typical recombinant expression yields approximately 100 mg of luciferase from 200 mL of bacterial culture grown using lactose auto-induction with pET plasmids. This quantity suffices for numerous experiments.
Use of crude E. coli lysates expressing luciferase avoids purification steps and suits many applications where ultra-high purity is unnecessary. Such methods simplify preparation and reduce cost.
As an alternative, natural luciferase extraction is possible from dissected firefly lanterns, though this source depends on accessibility.
Additionally, research plasmids coding for luciferase are frequently shared among laboratories, facilitating in-house production. This openness supports academic study and experimentation.
Summary of Key Points
- Firefly luciferase with 0.5 mM luciferin and 1 mM ATP produces visible light in vitro at enzyme concentrations between 0.03 and 0.1 mg/mL.
- Bioluminescence color varies by luciferase type; firefly emits green-yellow light.
- Luminescence is usually not visible with the naked eye under ambient light for other luciferases without darkroom settings.
- Live E. coli expressing firefly luciferase emit visible light upon addition of luciferin, useful for demonstration and research.
- Luciferin and ATP reagents are commercially available but costly; recombinant production in E. coli is a cost-effective alternative.
- Reaction buffer optimization includes substrate concentrations and additives like BSA and coenzyme A to enhance performance.
What concentrations of firefly luciferase and luciferin are needed to see light without a darkroom?
Using 0.03-0.1 mg/ml firefly luciferase with 0.5 mM luciferin and 1 mM ATP in the reaction buffer produces light visible even under ambient light. Lower concentrations can still produce a glow visible in darkness.
Can live *E. coli* produce visible bioluminescence with luciferase?
Yes, live *E. coli* expressing firefly luciferase emit visible light when treated with luciferin. The light appears as glowing blobs and streaks in the dark, useful for “glowing bacterial art.”
Is it necessary to purify luciferase for bioluminescence experiments?
No, crude *E. coli* lysates containing luciferase are often sufficient. Purification is optional and commercial luciferase is costly, so expressing luciferase in bacteria is a practical alternative.
What color of light does firefly luciferase emit during bioluminescence?
Firefly luciferase typically emits green to yellow-green light. Some variants can produce light shifting towards red wavelengths.
Are there additives to improve luciferase assay brightness or glow duration?
Yes, adding 1 mg BSA can enhance brightness, and coenzyme A improves signal strength and prolongs glow time in luciferase reactions.
How expensive are the reagents luciferin and ATP for bioluminescence assays?
Luciferin costs around $250 per gram, enough for multiple liters of solution. ATP is cheaper, about $54 per gram, making the reagents moderately expensive for experiments.




Leave a Comment