Is Latent Heat the Reason Behind Specific Heat Capacity?
Latent heat is not the reason behind specific heat capacity. They represent different thermal processes and physical phenomena. Latent heat occurs during a phase change at constant temperature, while specific heat capacity involves temperature change without a phase transition.
Defining Latent Heat and Specific Heat Capacity
Latent heat refers to the heat absorbed or released during a phase change such as melting or vaporization. This process happens without temperature change. Specific heat capacity is the amount of heat required to raise the temperature of a unit mass of a substance by one degree Celsius. This heat adds to sensible heat, causing temperature change.
How Materials Store Energy and Affect Heat Capacity

The variation in heat capacity among materials arises from the ways they store energy. Heat can raise atomic vibrations and other molecular motions.
- Vibrational energy increases molecular bond lengths, storing energy as potential energy.
- Materials with more energy storage modes require more heat to increase temperature.
Degrees of Freedom Explain Heat Capacity Differences
Specific heat capacity links closely to the degrees of freedom of molecules. Degrees of freedom include translational, rotational, and vibrational motions that absorb thermal energy.
For example, diatomic gases have higher heat capacities than monatomic ones because they exhibit rotational movements too. This leads to more ways to store energy, raising the heat capacity.
Relationship Between Latent Heat and Heat Capacity
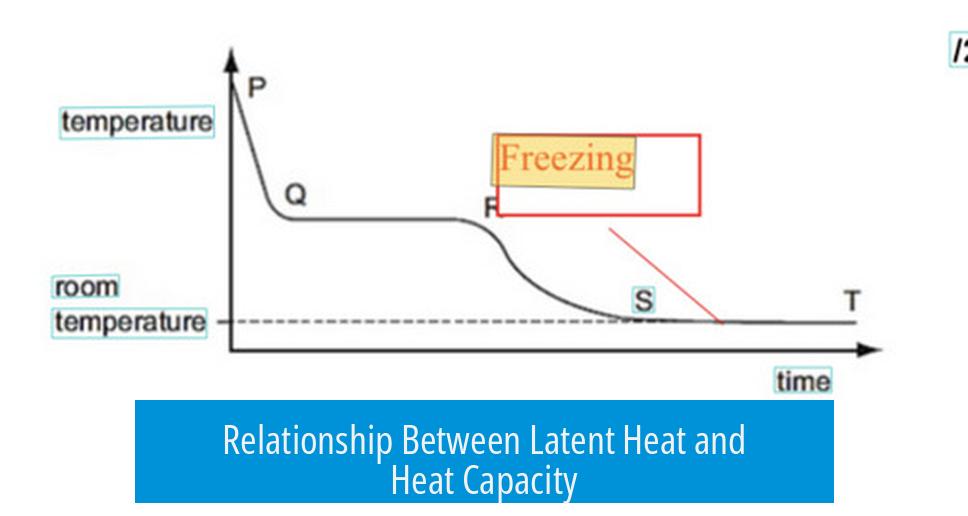
While latent heat and specific heat capacity differ, they connect mathematically. Latent heat equals the heat difference between two phases at the same temperature.
This energy difference can be approximated by integrating the specific heat capacity over temperature, from absolute zero to the phase change point.
Thus, substances with high liquid-phase heat capacity and low solid-phase heat capacity tend to have high latent heat of fusion. This relationship evidences an indirect but not causal link.
Key Takeaways
- Latent heat and specific heat capacity involve different processes—phase change vs. temperature change.
- Heat capacity depends on molecular energy storage modes and degrees of freedom.
- Latent heat relates to the energy difference between phases, calculable from specific heat capacities.
- Latent heat does not cause specific heat capacity but is connected through thermodynamic relationships.
Is Latent Heat the Reason Behind Specific Heat Capacity?

Short answer? No, latent heat is not the reason behind specific heat capacity. They are related concepts in thermal physics but fundamentally distinct. Understanding why materials heat up or change state takes a bit of unpacking, so stick with me.
First off, let’s clarify the main players: latent heat and specific heat capacity. Though their names sound cozy together, they aren’t siblings but distant cousins in the family of thermodynamics.
What Exactly Are Latent Heat and Specific Heat Capacity?
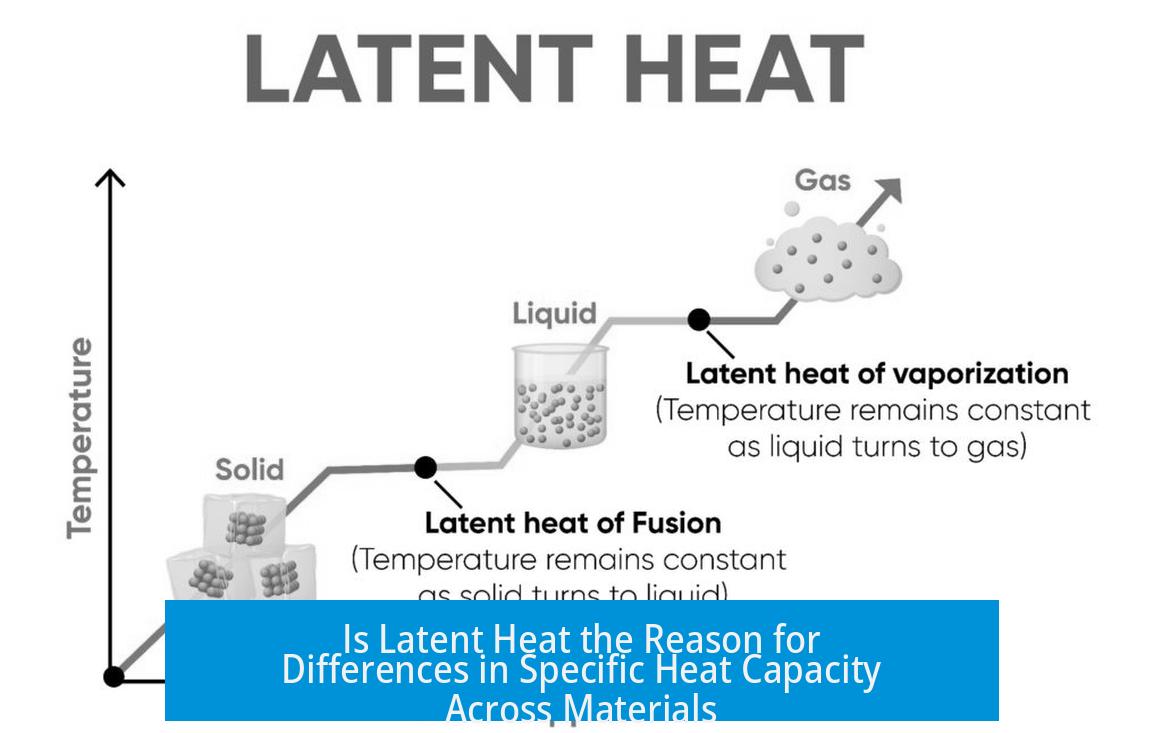
Latent heat is the heat energy absorbed or released during a phase change, like ice melting or water boiling, but—and this is key—the temperature stays constant during this process. Think about melting ice: heat energy flows in, but the thermometer doesn’t budge until it’s completely melted.
Specific heat capacity, on the contrary, measures how much heat energy it takes to change a substance’s temperature by one degree. It’s all about sensible heat—the heat you can “sense” or measure as temperature change.
So, latent heat is about constant temperature with changing states (solid to liquid or liquid to gas), while specific heat capacity deals with temperature changes within one phase. This difference by itself tells you latent heat cannot be the reason for specific heat capacity, because they describe different heat transfers.
Why Do Different Materials Have Different Specific Heat Capacities?
Great question! Here’s where things get interesting. Different materials don’t just heat up the same way because of latent heat; rather, they have different heat capacities because they store energy differently.
Imagine a tiny energy vault inside each molecule. Some materials have a lot of ways—like multiple locks and compartments—to store energy. Others have fewer. When you heat these materials, the energy can be stored in multiple forms:
- Vibrational energy: Atoms jiggle and vibrate more intensely.
- Rotational and translational energy: Molecules can spin and move around.
- Potential energy in molecular bonds: Bonds stretch and store energy without breaking.
Because of these variations, materials require different amounts of heat to increase their temperature. The more options for storing energy, the more heat you need. For instance, water has a pretty high specific heat capacity because the hydrogen bonds shuffle energy around in plenty of ways.
Degrees of Freedom: The Secret Behind Heat Capacity
Physics nerds, this one’s for you. Heat capacity aligns closely with the concept of degrees of freedom—basically, the number of ways molecules can move or vibrate.
Consider a single atom gas versus a diatomic gas like oxygen. The diatomic molecule can rotate, vibrate, and move in ways a lone atom cannot. That means it can absorb more energy before its temperature rises, which translates to a higher specific heat capacity.
In essence, the specific heat capacity depends on how many “energy storage options” the particle or molecule has. This idea is grounded in statistical mechanics, where the math beautifully backs up the physical intuition.
So, How Does Latent Heat Relate to Specific Heat Capacity?
While latent heat doesn’t cause specific heat capacity, they share an intriguing relationship. The latent heat between phases—say, melting or boiling—is essentially the difference in heat content between those two phases at the same temperature.
You can estimate the latent heat by integrating the specific heat capacity of the substance from absolute zero (0 K) up to that temperature. To put it simply, the energy needed to change phase equals the accumulated energy cost of heating the substance to that point, plus the extra “jump” during the transition.
Here’s a fun twist: materials with a high specific heat capacity in one phase and a low one in another tend to have a larger latent heat. For example, water liquid’s specific heat is quite high, while ice’s is lower. This difference contributes to water having a significant latent heat of fusion (melting).
Putting It All Together: What’s the Takeaway?
Latent heat is not the underlying cause of specific heat capacity. They describe different thermal processes: latent heat handles phase changes at constant temperature, while specific heat capacity manages temperature changes within a phase.
The heat capacity nuances come from how molecules store energy—through various degrees of freedom like vibration, rotation, and translation. Different materials and phases have different capacities, depending on what energy storage “tools” their molecules have.
But if latent heat doesn’t cause specific heat capacity, why does it seem connected?
Because the total energy a substance carries depends partly on how much energy it absorbed during heating (tracked by specific heat capacity) and partly on energy involved in phase change (latent heat). These quantities interplay but with distinct roles and mechanisms.
Practical Perspective: Why Does This Matter?
This distinction helps engineers and scientists design better materials and energy systems. Want a coolant that absorbs heat without heating up quickly? Look for a material with high specific heat capacity. Need phase change materials for thermal storage? Then latent heat capacity is your star.
Understanding the roots of specific heat capacity also opens the door to advancements in chemistry, physics, and material science. It sparks innovations in everything from climate control technologies to electronics cooling.
Final Food for Thought
Does the difference in degrees of freedom affect how we cook? Absolutely! Water’s high heat capacity means it heats more slowly than oil, meaning pasta boils longer in water, not just because of volume but molecular quirks behind the scenes.
Next time you watch ice melt or a pot simmer, appreciate these silent thermodynamic dances. From molecular vibration to energy jumps at melting points, the heat journey is a story of physics, not magic—latent heat and specific heat capacity each playing their own, unique roles.
Is latent heat the reason behind specific heat capacity?
No, latent heat and specific heat capacity are different concepts. Latent heat involves heat absorbed or released during a phase change at constant temperature, while specific heat capacity relates to heat required to change temperature.
How does heat capacity differ between materials?
Materials have different heat capacities because they store energy differently. More ways to store energy means more heat is needed to raise temperature. Vibrations and molecular bond expansions affect this storage.
What role do degrees of freedom play in specific heat capacity?
Degrees of freedom refer to ways particles can store energy. More degrees allow storing more energy, raising heat capacity. For example, molecules that rotate and vibrate have higher heat capacities than single atoms.
Can latent heat be calculated using specific heat capacity?
Latent heat can be estimated by integrating specific heat capacity over temperature, comparing energy between phases. High heat capacity differences between states often mean higher latent heat.




Leave a Comment