Endothermic but Exergonic Reactions: Clear Examples and Explanation
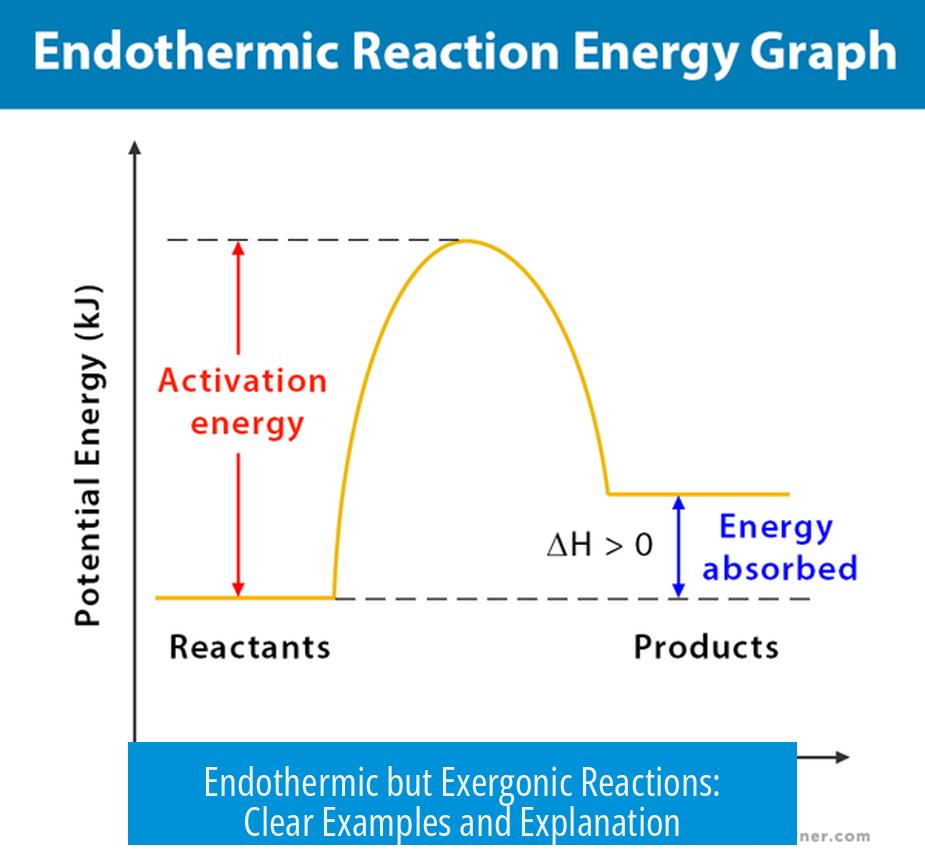
Some reactions absorb heat from their surroundings (endothermic) yet proceed spontaneously because the overall free energy change is negative (exergonic). This means while they need energy input, the total balance of enthalpy (ΔH) and entropy (ΔS) changes favors the reaction’s occurrence. Key examples include certain dissolutions, phase changes, and polymerizations.
Basic Thermodynamics: Understanding Endothermic and Exergonic
Reactions that are endothermic have a positive enthalpy change (ΔH > 0). They absorb heat. Exergonic reactions have a negative Gibbs free energy change (ΔG < 0), meaning they occur spontaneously under constant temperature and pressure.
The Gibbs equation:
ΔG = ΔH − TΔS
where:
- ΔH = enthalpy change
- T = temperature (Kelvin)
- ΔS = entropy change
If ΔH is positive but TΔS is larger and positive (large entropy gain), then ΔG can be negative, making the reaction spontaneous despite absorbing heat.
1. Dissolving Salts with Positive Enthalpy of Mixing
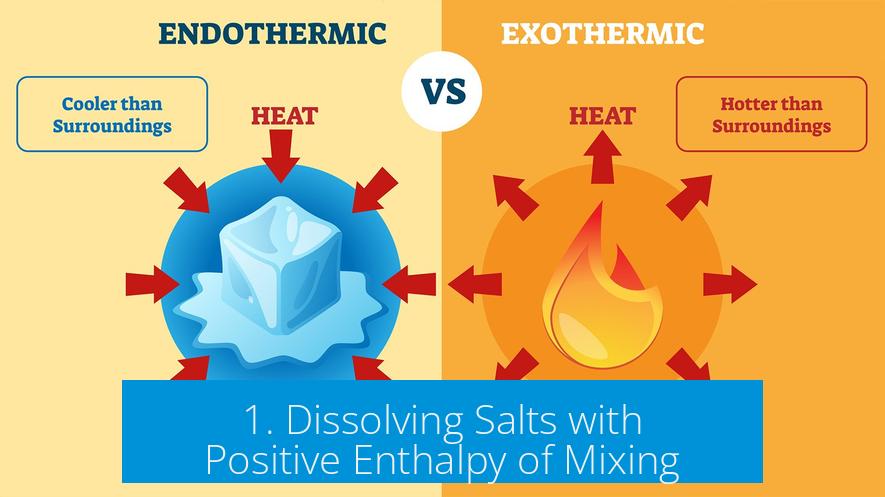
Some salts absorb heat when dissolving—this means their dissolution is endothermic (positive ΔH). Yet, these salts dissolve spontaneously due to a large increase in entropy in the system.
- Example: Dissolving potassium chloride (KCl) or ammonium nitrate (NH4NO3) in water.
- Heat is absorbed, causing the solution container to feel cold.
- The breaking of ionic lattice, hydration of ions, and increase in disorder in the solution contribute to a large positive ΔS.
- This entropy increase offsets the positive ΔH, resulting in negative ΔG.
This makes the dissolution process spontaneous even as it cools its surroundings. The entropy gain comes from the many possible arrangements of solvated ions compared to the ordered solid salt lattice.
2. Dissolving Ammonium Chloride in Water
Dissolution of ammonium chloride (NH4Cl) in water is a known endothermic yet spontaneous process.
- It absorbs heat, lowering the temperature of the solution.
- This is why the mixture feels cold after dissolution.
- Entropy increases because solid salt ions become dispersed in solution.
- Despite positive ΔH, the entropy gain sufficiently compensates, leading to a negative ΔG.
This effect is utilized in instant cold packs where ammonium nitrate or ammonium chloride dissolves endothermically, providing cooling.
3. Ring-Opening Polymerizations with Entropic Penalties
Ring-opening polymerizations demonstrate cases where enthalpy and entropy compete intricately.
| Aspect | Effect |
|---|---|
| Ring strain relief | Exothermic (negative ΔH) |
| Polymer formation (order increase) | Entropic penalty (decrease in ΔS) |
However, depending on the monomer:
- Some polymerizations absorb heat overall (endothermic ΔH) due to less ring strain or other energetic costs.
- Formation of an ordered polymer chain lowers entropy (negative ΔS).
- The balance between enthalpy and entropy and the temperature determines if ΔG is negative.
Under certain conditions, ring-opening polymerizations are endothermic yet exergonic, driven by suitable entropy-enthalpy interplay.
4. Boiling and Evaporation
Phase changes such as boiling and evaporation require absorption of significant heat energy.
- Heat converts liquid molecules into a gas phase (endothermic: positive ΔH).
- Molecules gain more freedom and disorder in gas phase versus liquid (entropy increases greatly).
- At a given temperature and pressure, the entropy term (TΔS) overcomes the heat input, yielding negative ΔG.
This explains why evaporation or boiling occurs spontaneously when heating a liquid to its boiling point:
- The process absorbs heat but proceeds on its own.
- Evaporation cools surfaces (sweat evaporating cools skin) because heat is absorbed from surroundings.
Explanation of Sweating Phenomenon
Sweating cools the body using endothermic evaporation. Despite requiring heat, the increase in molecular disorder in vapor phase drives the reaction, making it exergonic overall.
Summary Table: Endothermic but Exergonic Examples
| Example | Energy Feature | Reason for Spontaneity |
|---|---|---|
| Dissolving salts with positive enthalpy | Absorbs heat on dissolution | Large increase in entropy from ion dispersion |
| Dissolving ammonium chloride | Endothermic dissolution | Favorable entropy increase from mixed ions in solution |
| Ring-opening polymerizations (sometimes) | Endothermic due to polymer formation | Competition of enthalpy and entropy; balance allows spontaneity |
| Boiling/Evaporation | Heat absorbed to change phase | Huge entropy gain from phase change to gas |
Key Takeaways
- Endothermic reactions absorb heat but can still be spontaneous if entropy increase is sufficient.
- Gibbs free energy change (ΔG) determines reaction spontaneity, combining enthalpy and entropy effects.
- Dissolution of certain salts and ammonium chloride absorbs heat but increases solution entropy, driving spontaneity.
- Boiling and evaporation are endothermic yet spontaneous because of large entropy gain in vapor phase.
- Certain ring-opening polymerizations balance enthalpy and entropy changes so that some are endothermic but exergonic.
What Are Some Examples of Reactions That Are Endothermic, but Still Exergonic?
In a nutshell, some reactions absorb heat (endothermic) yet happen spontaneously (exergonic) because the increase in disorder, or entropy, compensates for the heat taken in. Sounds confusing at first? Let’s unpack this fascinating chemistry puzzle with concrete examples and clear explanations.
We usually think of reactions that absorb heat as ‘needing energy’ and thus non-spontaneous. But thermodynamics throws a curveball by considering entropy and Gibbs free energy (ΔG = ΔH – TΔS). If the entropy (disorder) increase is big enough, it can drive the reaction forward even if it sucks in heat. Let’s dive into some real-life and lab-taught examples.
Dissolving Salts with Positive Enthalpy of Mixing
Imagine dumping salt into water and watching the solution get cooler. Weird, right? This happens with salts that have a positive enthalpy of mixing. The process absorbs heat from the water, lowering the temperature (endothermic). But the salt still happily dissolves because the molecules in the solution gain so much freedom they create a huge entropy boost.
This powerful boost in disorder makes the reaction’s overall free energy negative—meaning it’s spontaneous—despite needing heat.
“One example is dissolving salts with a positive enthalpy of mixing. The reaction needs heat (so it cools down), but the reaction still occurs on its own, because the solution has higher entropy.”
So next time your salted ice water feels cooler, appreciate the subtle battle between heat and molecular chaos causing it.
Dissolving Ammonium Chloride in Water
Ammonium chloride is a chemist’s classic show-off for endothermic yet spontaneous reactions. When you toss this white crystalline salt into water, the container cools down because the salt sucks heat during dissolution.
Despite this, the salt dissolves completely without added pressure or fuss.
Why? Like the previous salt example, the entropy jump—the random mix of ions in water—increases so much that it drives the process forward, outweighing the heat absorbed.
“Dissolving ammonium chloride in water is quite endothermic but proceeds spontaneously due to favorable entropy changes.”
It’s like the ions are throwing a molecular party, spreading out to reduce favorability of sticking together and thus increasing disorder.
Ring-Opening Polymerizations with an Entropic Twist
This one requires a bit more thought but stick with me because it challenges common assumptions.
In ring-opening polymerizations, cyclic monomers “open” their rings to connect and form long polymers. Usually, breaking the ring releases strain and releases energy, making the enthalpy (ΔH) negative or exothermic. But here’s the catch—in some cases, the process can actually be endothermic (absorb heat) while still being spontaneous.
How? Because forming a structured polymer chain reduces entropy—monomers lose freedom by linking together, creating an ‘entropic penalty.’
The final spontaneity depends on the delicate balance between energy absorbed and entropy lost or gained. Some monomers have enough ring strain that even with heat absorbed, the reaction remains exergonic.
“Some ring-opening polymerizations can be endothermic yet exergonic depending on the balance of ΔH and ΔS. While breaking ring strain typically releases energy, the formation of an ordered polymer chain can reduce entropy.”
This shows that spontaneity isn’t just about heat—it’s about balance.
Boiling and Evaporation: Everyday Endothermic Exergonic Processes
Have you ever spilled rubbing alcohol on your skin and felt a chill? Or wondered how sweating cools you down on a hot day?
Boiling and evaporation processes suck in heat (endothermic). Heat energy breaks intermolecular attractions to convert liquid to gas. Yet, evaporation happens spontaneously at room temperature.
Why? Because vapor molecules jump freely and independently, greatly increasing the system’s entropy. This jump in disorder overpowers the heat absorbed.
“Boiling/evaporating is an endothermic, exergonic process. This explains why sweating cools you down—it absorbs heat while increasing entropy.”
So evaporation feels cold because it literally takes heat away, but it happens spontaneously because disorder wins.
Why Does This Matter?
Understanding that some **endothermic reactions are still exergonic** helps in many areas—from material science to biology.
- Designing better detergents: Salt dissolution effects impact cleaning efficiency and temperature.
- Pharmaceuticals: Polymerizations with controlled kinetics and energy profiles.
- Environmental science: Understanding phase changes like evaporation guides climate and weather predictions.
Moreover, it reminds us to think beyond just heat. Chemistry often dances on the line between energy and disorder—like a carefully balanced tango.
Common Question: Didn’t Exothermic Equal Spontaneous?
Not always. Spontaneity depends on the Gibbs free energy change (ΔG), which combines enthalpy (ΔH) and entropy (ΔS). A reaction can release heat (exothermic) but become non-spontaneous if it causes a big drop in entropy. Adsorption of gases onto surfaces is an example, where trapping the molecule reduces disorder enough to prevent spontaneous binding under some conditions.
Thus, both enthalpy and entropy work together to dictate whether a reaction happens on its own. Thermodynamics is like a seesaw—sometimes the weight is balanced by disorder, not heat.
Summary Table of Key Examples
| Example | Key Feature | Why Exergonic? |
|---|---|---|
| Dissolving Salts with Positive ΔH | Absorbs heat but solution disorder rises | Large increase in entropy (more disorder) |
| Dissolving Ammonium Chloride | Endothermic dissolution | Entropy increase from ions dispersing |
| Boiling / Evaporation | Heat absorbed to form vapor | Phase transition boosts entropy greatly |
| Selected Ring-Opening Polymerizations | Heat absorbed; polymer formation reduces entropy | Balance between ring strain release and ordering |
Final Thoughts
Endothermic but exergonic reactions prove that in the chemical world, it’s not just about energy but also about chaos. When things get more disordered, sometimes that alone is the green light for reactions to proceed—even if the heat budget looks bleak.
Next time heat is absorbed, ask yourself: Is the system getting more chaotic inside? If yes, it might just be an endothermic, exergonic reaction at play.
Curious to experiment? Try dissolving ammonium chloride in water and feel the temperature drop—your own mini chemical detective work!
What causes some endothermic reactions to be exergonic?
Endothermic reactions absorb heat, but if they increase entropy enough, the overall free energy change (ΔG) becomes negative. This drives the reaction to proceed spontaneously despite heat absorption.
Can dissolving salts be endothermic yet spontaneous?
Yes. Dissolving certain salts absorbs heat but increases the disorder of the system. This entropy gain outweighs the heat absorbed, making the process exergonic and spontaneous.
How does boiling water fit as an endothermic but exergonic process?
Boiling takes in heat to convert liquid to vapor, which is endothermic. However, the large increase in entropy from phase change makes boiling spontaneous at the boiling point.
Are all ring-opening polymerizations endothermic yet exergonic?
No. Only specific cases are. The balance of ring strain and entropy loss determines if ΔG is negative, so some ring-opening polymerizations absorb heat but still proceed spontaneously.
Why does dissolving ammonium chloride in water feel cold but still happen by itself?
It absorbs heat, causing cooling (endothermic), but the entropy increase in solution drives the reaction spontaneously, making it exergonic despite the temperature drop.



Leave a Comment