What Happened in the Research Lab? An Analysis

The image from the research lab shows a container of hydrochloric acid (HCl) that has undergone evaporation and chemical reactions due to improper storage. The HCl vapors escaped from the loosely sealed lid, reacted with atmospheric gases and possibly nearby ammonia or alkylamine bases, forming a crust of solid ammonium chloride (NH4Cl) and resulting in significant corrosion and safety hazards.
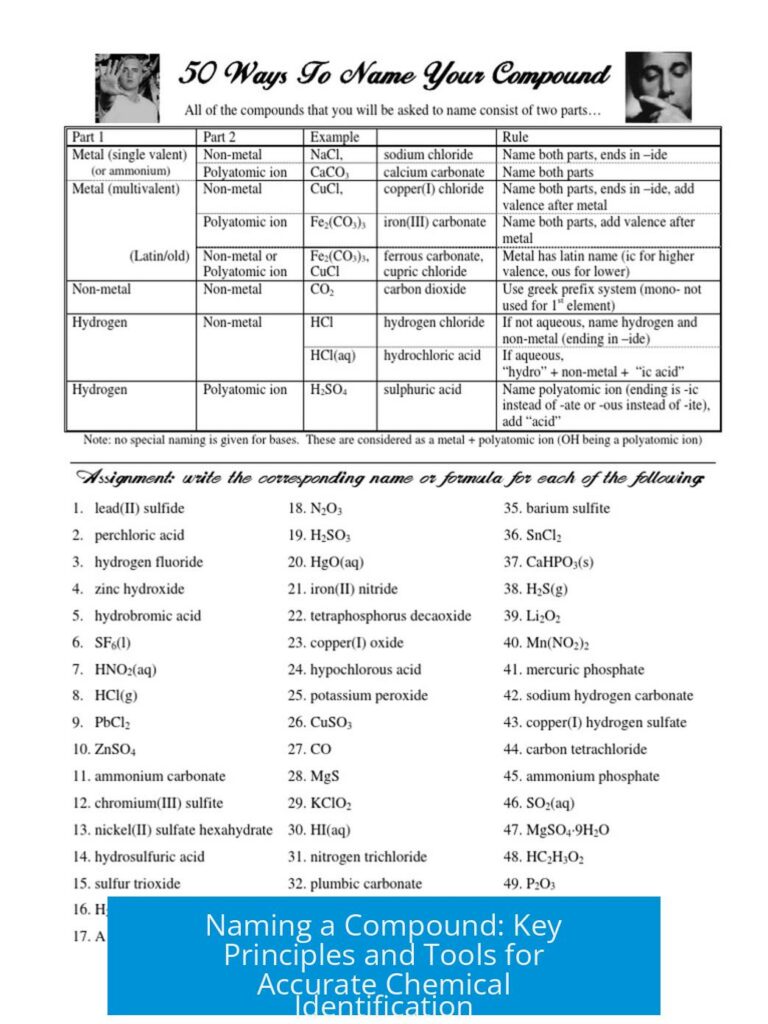
Chemical Reactions and Formation of Byproducts

The container initially held concentrated hydrochloric acid. Over time, the acid evaporated through a lid that was not fully sealed. The escaping HCl vapors then interacted with compounds in the surrounding environment. This interaction primarily involved ammonia or alkylamine bases often found stored nearby.
- Hydrochloric acid breaks down partially into hydrogen and chlorine gases before escaping the container.
- These gases react further with atmospheric components to produce visible crystalline deposits.
- Ammonia vapors react with HCl to form solid ammonium chloride (NH4Cl), a dense white crust, clearly visible on the container surface.
This salt formation acts as direct evidence of storage near incompatible chemicals like aqueous ammonia, triethylamine (TEA), or dialkylamines. Such proximity leads to salt deposits compromising container integrity and creating hazardous environments.
Observations on Lab Storage Practices and Safety Concerns

The condition of the bottle highlights common lapses in laboratory chemical management. Several key issues emerge:
- Improper Storage: The lid is not fully closed, allowing volatile acid vapors to escape.
- Inappropriate Container Choice: Concentrated HCl stored in plastic containers prone to degradation by acid.
- Mixed Chemicals: Storing strong acids near bases like ammonia or TEA encourages salt formation and corrosion.
- Old and Mislabeled Containers: The jug appears aged beyond recommended use, lacking current hazard labels. One container was incorrectly relabeled from methanol to isopropanol without updating toxicity warnings.
- Ventilation Issues: Likely inadequate air exchange allows corrosive vapors to accumulate.
- Structural Damage: A highly corroded shelf indicates acid vapor deposition, risking collapse and chemical spills.
These observations point toward negligence and poor laboratory discipline, which jeopardizes personnel safety and experimental integrity.
Safety Recommendations and Reactions
Upon discovering such a scenario, immediate and appropriate actions are essential:
- Evacuate the area promptly to avoid inhalation of corrosive or toxic vapors.
- Notify Environmental Health and Safety (EHS) personnel for proper containment, neutralization, or disposal.
- Dispose of acid waste into designated aqueous waste containers if trained to do so safely.
- Ensure incompatible chemicals like strong acids and bases are stored separately in dedicated, ventilated, and resistant solvent cabinets.
- Replace old chemical containers with freshly supplied, clearly labeled jugs adhering to safety standards.
- Improve ventilation to prevent vapor buildup and corrosive damage.
- Inspect and repair or replace corroded storage furniture to avoid accidents.
Additional Observations and Lab Culture Reflections

The incident invites reflection on broader laboratory practices. Instances of mislabeled chemicals, outdated containers, and storage mishaps appear frequent even in academic settings, sometimes reflecting systemic neglect or insufficient training.
Some researchers report encountering other dangerous or misidentified chemicals, such as thionyl chloride mistaken for diethyl ether, which could lead to severe consequences.
Despite the concerns, humorous remarks on the situation serve as reminders that chemistry requires continuous vigilance and adherence to safety protocols to avoid “forbidden milk” or “Sigma male energy evaporation” mishaps.
Summary of Key Points
- HCl vapors escaped a loosely sealed container and reacted with ammonia or alkylamines, forming solid ammonium chloride.
- Improper storage—old containers, poor labeling, unsuitable lids—caused chemical vapors and corrosion.
- Lab ventilation and storage furniture suffered damage due to acid vapor exposure.
- Immediate safety requires evacuation, notification of EHS, and proper waste disposal.
- Strong acids and bases must be stored separately in appropriate, corrosion-resistant cabinets.
- Proper labeling, container maintenance, and training reduce chemical hazards significantly.
What happened here? Insights from a Research Lab Visit
You walk into a research lab and notice a mysterious crust forming around a bottle labeled “HCl.” What’s going on here? Simply put, the hydrochloric acid has evaporated, mingled with airborne chemicals—particularly ammonia—and created solid ammonium chloride deposits on the container and shelf. But that’s just the tip of this chemical iceberg.
Let’s dissect this curious event piece by piece—because in laboratories, what looks like a simple mess often tells a deeper story about chemistry, safety, and human behaviors.
Unpacking the Chemical Drama: From HCl Vapor to Salt Crust
The core culprit is hydrochloric acid’s penchant for escaping its container, especially if it’s not sealed properly. Here, the lid wasn’t fully closed. This oversight allowed HCl vapors to sneak out. When these vapors meet ammonia, a classic chemical reaction happens: formation of ammonium chloride (NH4Cl), a solid white salt deposited visibly around the bottle.
Think of it as chemistry’s version of an uninvited party guest who spots their favorite snack and refuses to leave—leading to a mess. But instead of chips, it’s corrosive vapors reacting in the air.
Interestingly, the acid also partially decomposed into hydrogen and chlorine gases. These gases further combined with other atmospheric substances, intensifying the crust buildup and causing corrosion nearby. If you see a shelf weakening or metal parts turning flaky—you’re witnessing years of chemical neglect making their mark.
The Science Meets Sloppiness: Lab Storage Gone Wrong
The phenomena observed here are efficient reminders of how improper chemical storage can create hazardous conditions. Storing concentrated hydrochloric acid in plastic containers is frowned upon due to potential degradation over time. Additionally, the container in question looks old—possibly 8-10 years—which surpasses the recommended safe lifespan of five years. Lack of proper classification labels (CLP/GHS marks missing) signals either forgetfulness or outdated management.
And yes, the classic and avoidable blunder happened again—placing HCl beside aqueous ammonia or alkylamine bases like triethylamine (TEA) or dialkylamine. It’s almost like the saga of “never-neighbors.” These shouldn’t live next door. Yet, the “sigma male energy”—perhaps a nod to careless ownership—led to escape and reaction of vapors.
To spice things up, one bottle had its original “Methanol” label scratched out and replaced with “isoPROPANOL,” but the toxic symbol stayed. Is this laziness or a chemical disaster waiting to happen? Neither should pass reality checks in a professional lab.
Why Does This Matter? Ventilation and Safety Risks
Besides the chemical growth spurt on the shelf, ventilation (or the lack of it) plays a starring role. Poor or nonexistent airflow lets toxic vapors accumulate. Picture a confined chemical sauna where acid fumes freely wander—breathing that air isn’t exactly a spa treatment.
Shelf corrosion adds a physical risk: structures weaken and might collapse, causing spills, injuries, or even worse reactions. This is not the moment for a “hold my pipette” stunt.
What Should You Do If You Spot a Similar Situation?
- First, get out. Seriously. An “oh crap” reaction is warranted because toxic vapors might be present.
- Notify Environmental Health and Safety (EHS) authorities immediately for professional handling.
- If trained, neutralize the acid properly or dispose of it into the designated aqueous waste system.
- Place chemicals in well-ventilated, secure solvent cabinets—the unsung heroes of lab safety.
Always remember: safety protocols aren’t just bureaucracy but subtle life preservers.
Not Just a Chemistry Lesson: Lab Culture and Common Sense
Many labs, especially in academia, inherit quirky habits and occasional negligence. Stories emerge about mislabeled bottles, mysterious substances, or “forbidden milk” stored as if to test our sanity or health. These anecdotes aren’t funny when they involve toxic exposure or damaged experiments but do reflect a universal struggle between human error and scientific rigor.
Imagine mistaking thionyl chloride for diethyl ether. Not a rerun of a sci-fi thriller, but a genuine hazard that could turn a mundane lab day into chaos.
Does your lab have a “sigma male energy evaporation” bottle? Maybe not—but avoiding becoming *that* lab is crucial.
Final Thoughts: Lessons from Chemical Chaos
What happened here is a blend of chemistry in action and human oversight. The escape of HCl vapors, reaction with ammonia forming ammonium chloride salt crust, poor storage, and aging containers summed up an accident waiting to unfold. It’s both a cautionary tale and a peek into how lab environments can spiral without constant vigilance.
Next time you approach a chemical bottle, close the lid tight. Store incompatible chemicals apart. Check container age and labels. If you see corrosion or crust, question why it’s there. Because, in labs, chemistry isn’t just about reactions on paper—it’s the silent story told by what happens when we’re not paying attention.
Got weird chemical stories from your lab visits? Drop a comment below. Let’s keep labs safe and interesting—minus the hazardous surprises!
What caused the white crust seen around the chemical containers?
Hydrogen chloride vapors escaped from the bottle and reacted with ammonia or other amine bases nearby. This formed solid ammonium chloride, which appears as the white crust on the container.
Why was the chemical in the plastic container corroded and leaking?
The container was likely old and unsuitable for storing concentrated HCl. Plastic can degrade over time, especially if the lid isn’t sealed tightly, causing acid vapors to escape and corrode the surroundings.
What are the safety risks of storing HCl near amines or ammonia?
HCl vapors react with amine vapors to form solid salts, which can cause buildup and contamination. Improper storage like this also increases the risk of toxic fumes escaping, creating hazards for lab personnel.
Why is the labeling on these chemical containers a problem?
Some containers are mislabeled, showing old or incorrect names and hazard warnings. This leads to confusion and unsafe handling since the true contents and risks aren’t clearly communicated.
What immediate actions should be taken if such chemical deterioration is noticed?
Leave the area promptly to avoid exposure. Notify environmental health and safety personnel to handle neutralization and cleanup properly. Never try to fix the issue alone.
How can these storage issues be prevented in the lab?
Store chemicals in proper containers and locations, avoid placing incompatible chemicals nearby, maintain good ventilation, and regularly check labels and container conditions. Use solvent cabinets for corrosive substances.




Leave a Comment