The Circular Periodic Table of Elements
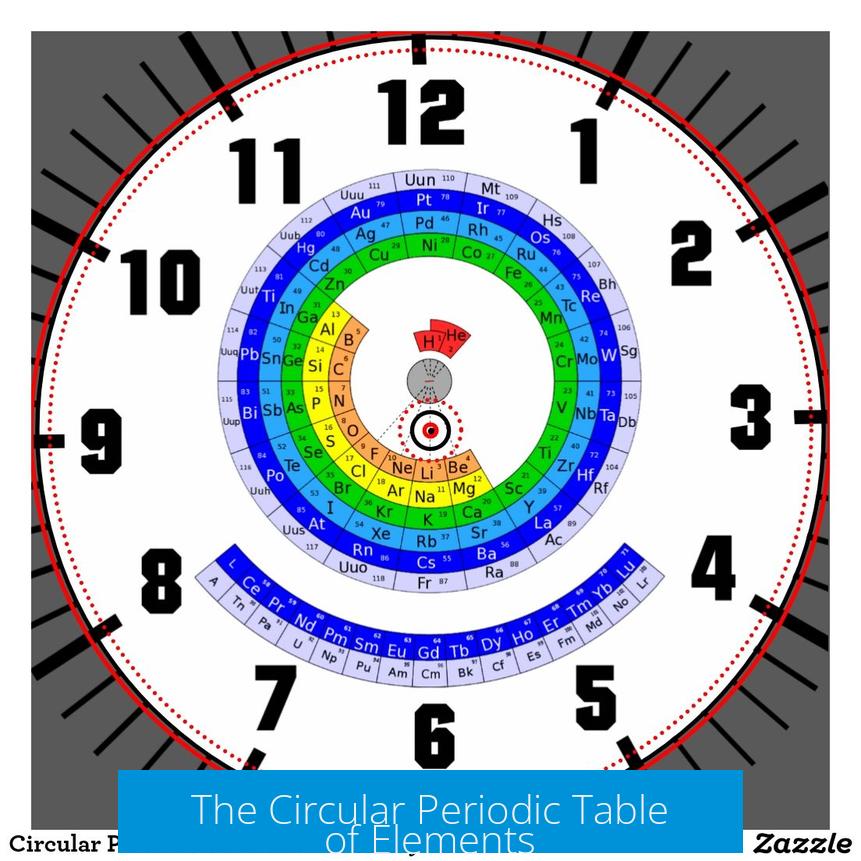
The Circular Periodic Table of Elements is a visually innovative representation of the chemical elements arranged in a circular layout rather than the traditional rectangular format. This design emphasizes periodic trends while adding an artistic and educational dimension, though it faces challenges related to practical usage and structural clarity. The circular format aims to portray electron shells and element groups in a way that aligns spatially with their electron configurations, offering a fresh perspective on element periodicity.
Design and Visual Features
The Circular Periodic Table features a sleek, modern design admired for its aesthetics. It uses concentric circles to represent electron shells, with elements arranged radially around the center, typically starting with hydrogen at either the top or bottom of the circle. Its visual appeal is marked by smooth, swooshing curves that connect periods, lending the table a dynamic and flowing appearance.
- The table’s design highlights the concept of periodicity by plotting elements along circular tracks that correspond to increasing principal quantum numbers (n).
- It often includes a “cliff” or gap representing the transition between different blocks of elements, such as between s and p blocks.
- The radial layout enhances the symmetry and gives the table an artistic touch comparable to fractal patterns, like those in the Mandelbrot set.
- Poster versions of the circular table are popular for classrooms and laboratories due to their attractive and clean visuals.
Despite its polished look, some users find the swirl or swoop connecting certain elements confusing. The transition between periods, especially from noble gases to alkali metals, appears visually ambiguous, leading to difficulty in immediately grasping horizontal periodic trends.
Labels and Notations
Accurate labeling is vital for any periodic table, and the circular format introduces new challenges and opportunities. Because the design highlights electron shells, labels commonly mark principal quantum numbers (n) around concentric circles. Yet, points of contention have arisen concerning subshell notations:
- Labels like “2*s*”, “3*s*” are often replaced by the clearer and scientifically correct “n=2”, “n=3”, etc., since “s” subshell info is only specifically relevant for particular groups.
- Group numbers may be added to the circular table to maintain a connection with standard classifications (Groups 1-18), aiding in element categorization and reference.
- Block labels (s, p, d, f) can be introduced either radially or tangentially to the circles to highlight electron subshell occupancy.
- Orientation changes, such as placing Hydrogen at the 12 o’clock position, have been proposed to improve intuitive reading and periodicity flow.
Structural Layout and Period Transitions
The circular format integrates element periods as concentric rings outward from the center. This spatial approach aligns with the quantum model of atoms, where electrons occupy shells further from the nucleus as the period number increases.
- The period transition, notably from helium to lithium, is represented as moving from one ring (shell) to the next outward ring, denoting a new principal quantum level.
- Some designs use a “slide” or swooping path to connect the end of one period to the start of the next, visually emphasizing electronic closeness rather than strict adjacency typical in tabular forms.
- However, this swoop has drawn critique as it distorts the cognitive perception of period order. It can mislead users to interpret the transition as moving “downwards” or backwards, contradicting electron shell progression.
- Suggestions include removing the swoop or enhancing the design with circular guide tracks to clarify period boundaries and aid quick element location.
Space allocation within the circular table sometimes results in uneven gaps, with empty zones particularly noticed in the right and central areas. These affect the visual flow, causing readers to focus non-uniformly and complicate scanning for certain elements.
Practicality and Usefulness
The Circular Periodic Table offers educational value by illustrating quantum shell structure and electronic relationships among elements. Many educators consider it a novel teaching aid that complements traditional periodic tables by framing element periodicity in a quantum context.
- Its circular form helps learners visualize electron shell progression naturally, which is harder to capture in rectangular tables.
- However, the unusual layout may hinder quick reference during laboratory work or chemical calculations where group and period information must be immediately accessible.
- The design requires familiarization; users not accustomed to this format might find it difficult to navigate or interpret element positions instantly.
- Thus, it is more suited for academic discussion, conceptual visualization, and artistic display than daily practical reference.
Variations of Periodic Tables and Physical Significance
Periodic tables have many forms, including spirals, wheels, and 3D models, each attempting to reflect element periodicity with varying emphases on physical or chemical properties. The circular table aligns somewhat with spiral periodic tables that plot elements in a swirling order based on increasing atomic number.
- Spiral tables emphasize continuity of atomic numbers but often sacrifice clarity in grouping elements by chemical properties.
- The circular format tries to preserve groupings while presenting a visual map of electron shell filling sequences.
- Critics argue that some design elements, like the swooping connections, lack direct physical significance and complicate interpretation.
- Despite these critiques, designs like the circular table stimulate fresh thinking on how to represent intrinsic quantum relationships among elements.
Traditional rectangular tables maintain dominance primarily for clarity, simplicity, and widespread acceptance. They balance chemical similarity, electronic configuration, and practical referencing effectively.
Element Grouping and Chemical Considerations
The circular table must still address complex chemical debates, especially about element placements in specific groups. Group 3 is a prime example, where confusion exists over whether lanthanum (La) and actinium (Ac) belong with scandium (Sc) and yttrium (Y) or the lanthanides and actinides.
| Element | Group 3 Placement Debate | Electron Configuration | Chemical Similarity |
|---|---|---|---|
| Sc (Scandium) | Agreed Group 3 element | [Ar] 3d1 4s2 | Similar to Y, La, Ac |
| Y (Yttrium) | Agreed Group 3 element | [Kr] 4d1 5s2 | Similar to Sc, La, Ac |
| La (Lanthanum) | Placement debated; some place in Group 3, others among lanthanides | [Xe] 5d1 6s2 | Shares properties with Sc and Y |
| Ac (Actinium) | Placement debated; mirrors La’s debate | [Rn] 6d1 7s2 | Similar chemical behavior to La, Sc, Y |
Adjustments in color coding in the circular table can emphasize block membership. For example, lutetium (Lu) and lawrencium (Lr) might be differently colored to indicate their d-block character instead of the f-block to reflect electron configurations properly.
Summary of Key Points
- The Circular Periodic Table arranges elements radially along concentric shells, linking quantum numbers (n) with period number.
- Its design offers a fresh visualization that highlights electronic structure over simple chemical grouping.
- Labeling improvements recommend using “n=” notations for shells and including group numbers for better clarity.
- Period transitions are illustrated as outward movement but the swooping lines between periods can confuse users.
- While visually attractive and educational, the circular table is less practical for quick reference than traditional tables.
- It plays a supportive educational role, especially for understanding electron shell filling and atomic structure.
- The group 3 element placement remains a debated topic; circular tables can integrate visual cues to reflect chemical similarities accurately.
- Overall, circular periodic tables expand conceptual frameworks but complement rather than replace standard rectangular formats.
What is the main design feature of the Circular Periodic Table?
The table arranges elements in a circular layout with concentric shells representing periods. It aims to visually link electron shells in an intuitive way. This layout highlights element relationships differently from the traditional rectangular table.
Why do some suggest changing the shell labels from “2s*, 3s*” to “n=2, n=3”?
The labels with “s” subshells apply mainly to the first two groups and can be confusing. Using “n=” followed by the shell number clarifies the period shell without implying specific subshell types.
How does the “swoop” or “slide” between periods affect understanding?
The swoop aims to show electronic similarity between elements like Neon and Sodium. However, some find it confusing as it suggests a downward movement conflicting with shell progression which actually goes outward or upward.
Is the Circular Periodic Table practical for everyday use?
Many users feel it’s more suited for teaching or visual appeal than daily reference. Its unique layout may challenge quick lookup but can aid conceptual understanding of electron shells.
How does this circular design handle Group 3 element placement?
The circular table faces the same debates as traditional tables about Group 3 composition. Some propose grouping Scandium and Yttrium with Lanthanum and Actinium due to similar electron configurations and chemistry.




Leave a Comment