Is CO2 a Triple Bond?
CO2 does not have a triple bond; it features two double bonds between carbon and oxygen atoms that satisfy the octet rule and best describe the molecule’s bonding. This distinction contrasts with molecules like carbon monoxide (CO), which do possess a triple bond. Examining bonding in CO2, resonance structures, and comparisons with CO clarifies the true nature of carbon dioxide’s bonds.
Bonding in Carbon Dioxide (CO2)
Carbon dioxide consists of one carbon atom double bonded to two oxygen atoms. Each double bond involves sharing four electrons, fulfilling the octet for carbon and oxygen. The widely accepted Lewis structure depicts these two double bonds symmetrically.
- CO2’s carbon forms two double bonds, one with each oxygen.
- Each atom attains a complete octet, stabilizing the molecule.
- No triple bonds exist between carbon and oxygen in CO2.
Some confusion arises from alternative resonance forms which substitute one double bond for a triple bond coupled with a single bond on the other side. However, these forms carry formal charges that destabilize the molecule. The optimal resonance form minimizes these charges, favoring two double bonds in CO2.
Resonance Structures and Limitations of Lewis Models
Lewis structures serve as simplified models for bonding. In CO2, resonance forms include:
- Two double bonds (preferred structure) with neutral atoms.
- One triple bond and one single bond with charged atoms (less stable).
These models do not perfectly represent the true electron distribution. Electron density in CO2 delocalizes over the molecule, favoring the electronegative oxygen atoms. The actual bonding is better described by molecular orbital theory, showing partial bond character between carbon and oxygens.
Thus, resonance is a tool to understand bonding but does not imply that the molecule constantly switches between distinct bonding states. The triple bond resonance forms in CO2 exist theoretically but are neither dominant nor accurate descriptors of the molecule’s bonding framework.
Difference Between CO2 and Carbon Monoxide (CO) Bonding
| Molecule | Bond Type | Number of Bonds Between C and O | Formal Charges |
|---|---|---|---|
| CO2 (Carbon dioxide) | Double bonds | Two double bonds (one per oxygen) | Minimal; atoms are neutral |
| CO (Carbon monoxide) | Triple bond | One triple bond between C and O | Small formal charges, stabilized by bond |
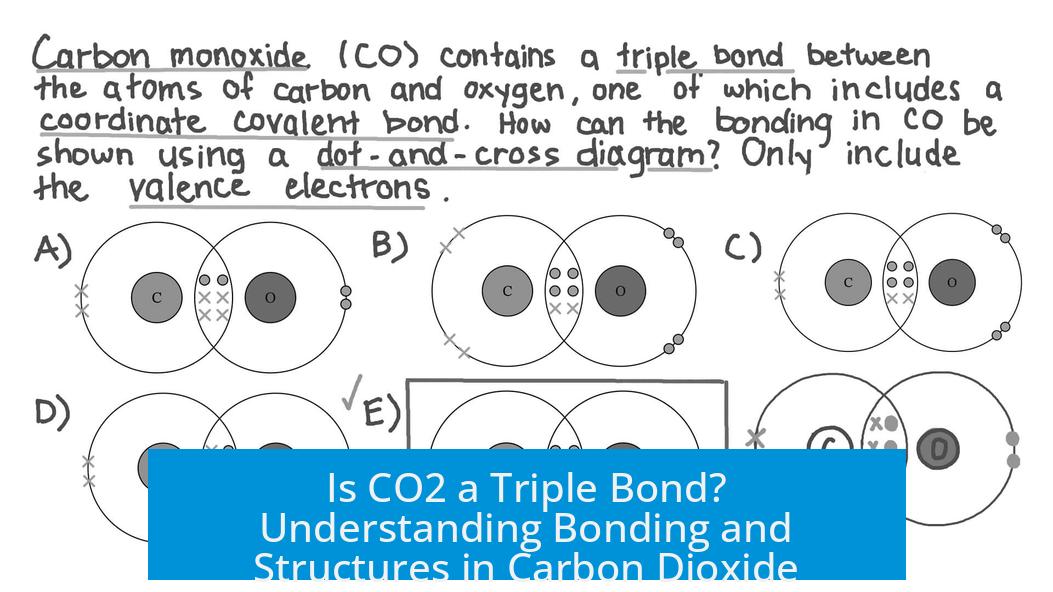
The carbon monoxide molecule features a triple bond between one carbon and one oxygen atom. This triple bond entails one sigma and two pi bonds, creating a strong connection and satisfying octets. CO’s bonding is fundamentally different from CO2, where the central carbon bonds to two oxygens with double bonds.
Critique of Some Misleading Explanations
Some textbooks or informal sources incorrectly portray CO2 as having triple bonds. Such inaccuracies stem from a misunderstanding of basic bonding principles or misuse of Lewis structures. These sources may:
- Show CO2 with triple bonds on both sides, which is chemically invalid.
- Misrepresent octet fulfillment or electron counts.
- Fail to recognize resonance preference and charge minimization.
It is essential to rely on established, peer-reviewed chemistry textbooks or validated resources for correct bonding representations. Faulty models can mislead students and obstruct proper understanding.
Key Takeaways
- CO2 contains two double bonds between carbon and oxygen atoms, not triple bonds.
- Resonance forms with triple bonds exist but are less stable and not the primary structure.
- Electron distribution in CO2 is delocalized; Lewis structures are simplified models.
- CO differs by featuring a true triple bond between carbon and oxygen.
- Careful evaluation of chemistry sources is vital to avoid misconceptions.
Does CO2 have a triple bond between carbon and oxygen?
No, CO2 does not have a triple bond. It has two double bonds between the carbon and each oxygen atom.
Why do some resonance structures of CO2 show a triple bond?
Resonance structures sometimes depict one triple bond and one single bond in CO2, but these forms are less stable due to unfavorable charge distribution.
How is the bonding in CO2 different from carbon monoxide (CO)?
CO has a true triple bond between carbon and oxygen, while CO2 has two double bonds with resonance and no triple bonds.
What role do Lewis structures play in representing CO2 bonds?
Lewis structures are simplified models that show bonding but do not capture the true electron distribution. They help visualize double bonds in CO2 but are not perfectly accurate.
Are books claiming CO2 has triple bonds reliable?
Books that state CO2 has triple bonds often contain errors. It is best to rely on established textbooks that correctly show CO2 with double bonds.




Leave a Comment