How Do Acids Work? Why Do They Burn and Break Down Substances?
Acids work primarily by releasing hydrogen ions (protons, H+) when dissolved in water, producing reactive hydronium ions. These protons attach to molecules, often at negatively charged or polar sites, increasing molecular reactivity and breaking chemical bonds. This protonation leads to the breakdown of substances and causes the burning sensation usually associated with acids.
1. Nature and Composition of Acids
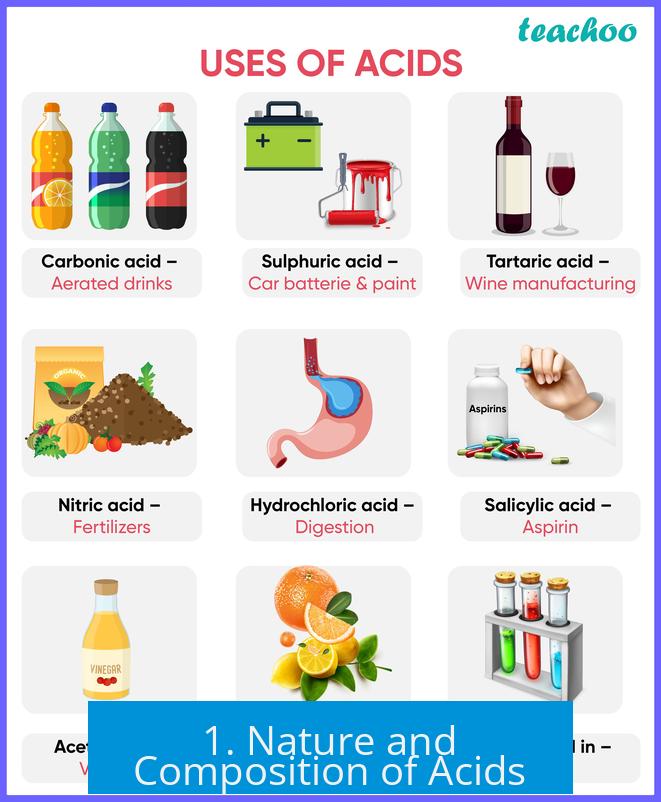
1.1 Presence of Hydrogen Ions

Acids contain one or more hydrogen atoms that can release a positive hydrogen ion, known as a proton (H+), into solution.
Examples include hydrochloric acid (HCl), which dissociates into H+ and Cl-, and sulfuric acid (H2SO4), which produces two protons and a sulfate ion (SO42-).
The proton is highly reactive and readily interacts with other molecules.
1.2 Dissociation in Water and Hydronium Ion Formation
When acids dissolve in water, they release protons into the surrounding solution.
These free protons associate with water molecules, forming hydronium ions (H3O+), increasing the solution’s reactivity.
The acid dissociation constant (Ka) measures how readily an acid releases protons; a high Ka means more protons are available.
2. Mechanism of Acid Reactions: Protonation and Breakdown
2.1 Role of Hydrogen Ions in Breaking Down Substances
The free protons generated bind to negatively charged or electron-rich sites on molecules, a process called protonation.
Protonation alters molecular structure, increasing chemical reactivity and making bonds more vulnerable to cleavage.
For example, acids target unsaturated bonds or polar regions, destabilizing structures and leading to breakdown.
2.2 Chemical Stability and Proton Donation
Acids can donate protons without destabilizing their own structure significantly.
The resulting conjugate base (anion) after proton loss is more energetically stable, facilitating continual proton release.
This stability drives acidic behavior and supports ongoing reactions in solution.
3. Why Acids Burn and Break Down Substances
3.1 Increased Reactivity by Protonation
Protonation creates a condition favorable for reactions, magnifying reaction rates considerably.
The burning sensation results from acids rapidly reacting with skin proteins and nerve endings.
Protons attack molecular sites, disrupting bonds and damaging tissues, causing a burning or stinging feeling.
3.2 Interaction with Negatively Charged and Polar Sites
Acids target electron-rich regions within molecules by the hydrogen’s interaction with negative charges or lone electron pairs.
The proton’s positive charge attracts electrons, weakening bonds and allowing the acid to “break down” complex molecules into smaller parts.
4. Acid Strength and Effectiveness in Reactions
4.1 Acid Dissociation Constant (Ka) and Proton Availability
The acid dissociation constant (Ka) quantifies equilibrium between protonated and unprotonated forms in solution.
Stronger acids have higher Ka values and release more free protons, increasing their ability to react and break down substances.
4.2 Relevance of Acid Strength in Material Breakdown
Stronger acids with more free H+ ions accelerate protonation reactions, leading to faster degradation of materials like metals, proteins, and polymers.
Weaker acids yield fewer free protons, resulting in comparatively slower or less extensive reactions.
5. Additional Chemical Characteristics of Acids
5.1 Interaction with Bases
Acids neutralize bases by combining free H+ ions with hydroxide ions (OH-), forming water and often releasing heat.
This exothermic reaction exemplifies acid-base chemistry and can be vigorous, causing rapid energy release.
A common example is vinegar (acid) reacting with baking soda (base), creating bubbles of vaporized water and carbon dioxide.
5.2 Metal Dissolution by Strong Acids
Strong acids such as aqua regia dissolve metals by donating a high concentration of protons and generating reactive species.
These protons react with metal atoms, oxidizing them and breaking metallic bonds, facilitating dissolution.
6. Molecular Basis for Acid Behavior
6.1 Composition and Electron Interactions
Acids are molecules composed of hydrogen atoms bonded to an electronegative anion.
Because the anion attracts electrons more than the hydrogen, the hydrogen tends to be positively charged and thus reactive.
6.2 Proton Donation and Stability of Conjugate Base
Acids readily donate protons to achieve a more stable conjugate anion.
This energetic stability favors proton loss and promotes reactivity in acidic solutions.
Summary of Key Points
- Acids release protons (H+) in water, forming reactive hydronium ions.
- Protons bind to negatively charged or polar molecular regions, causing protonation.
- Protonation destabilizes molecules and breaks chemical bonds, leading to material breakdown.
- The acid dissociation constant (Ka) determines proton availability and acid strength.
- Strong acids with high Ka values induce faster and more extensive chemical reactions.
- The burning sensation from acids arises from rapid proton-mediated damage to biological tissues.
- Acids can neutralize bases in exothermic reactions, forming water and releasing heat.
- Metal dissolution by acids involves proton-induced oxidation and bond disruption.
How do acids cause materials to break down?
Acids release hydrogen ions (H+) that bond with negatively charged parts of molecules. This protonation changes molecular structure, making them reactive and leading to bond breaking and material breakdown.
Why do acids feel like they burn on skin?
The burning sensation is due to acids donating protons that react quickly with sensitive molecules in skin and tissues. This causes chemical changes and irritation, which feels like burning.
What role does the acid dissociation constant (Ka) play in acid strength?
Ka measures how readily an acid releases hydrogen ions in water. A higher Ka means more free protons, resulting in stronger acidity and greater ability to break down substances.
Why do only certain substances break down when exposed to acids?
Acids specifically react with molecules that have negatively charged or electron-rich areas. These areas attract the acid’s hydrogen ions, causing selective chemical reactions and breakdown.
How do acids interact with other chemicals to produce heat?
When acids meet bases, their hydrogen ions combine with hydroxide ions to form water. This reaction forms new bonds and releases heat, sometimes causing visible effects like bubbling.




Leave a Comment