Easiest Way to Identify Which Elements Are Cations and Anions
Determining whether an element forms a cation or an anion primarily depends on its metallic or nonmetallic nature. Metals tend to lose electrons and form positively charged ions, called cations. Nonmetals tend to gain electrons and become negatively charged ions, known as anions.
General Identification Rule

- Metals form cations. They lose electrons during ionic bonding.
- Nonmetals form anions. They gain electrons when forming ionic bonds.
This rule applies largely because of the differing electron configurations and tendencies of metals and nonmetals. Metals have fewer valence electrons which they lose to achieve a stable octet configuration. Nonmetals gain electrons for the same reason.
Charge Characteristics of Ions
- Cations carry a positive charge. This is due to electron loss (electron count < proton count).
- Anions carry a negative charge. Resulting from electron gain (electron count > proton count).
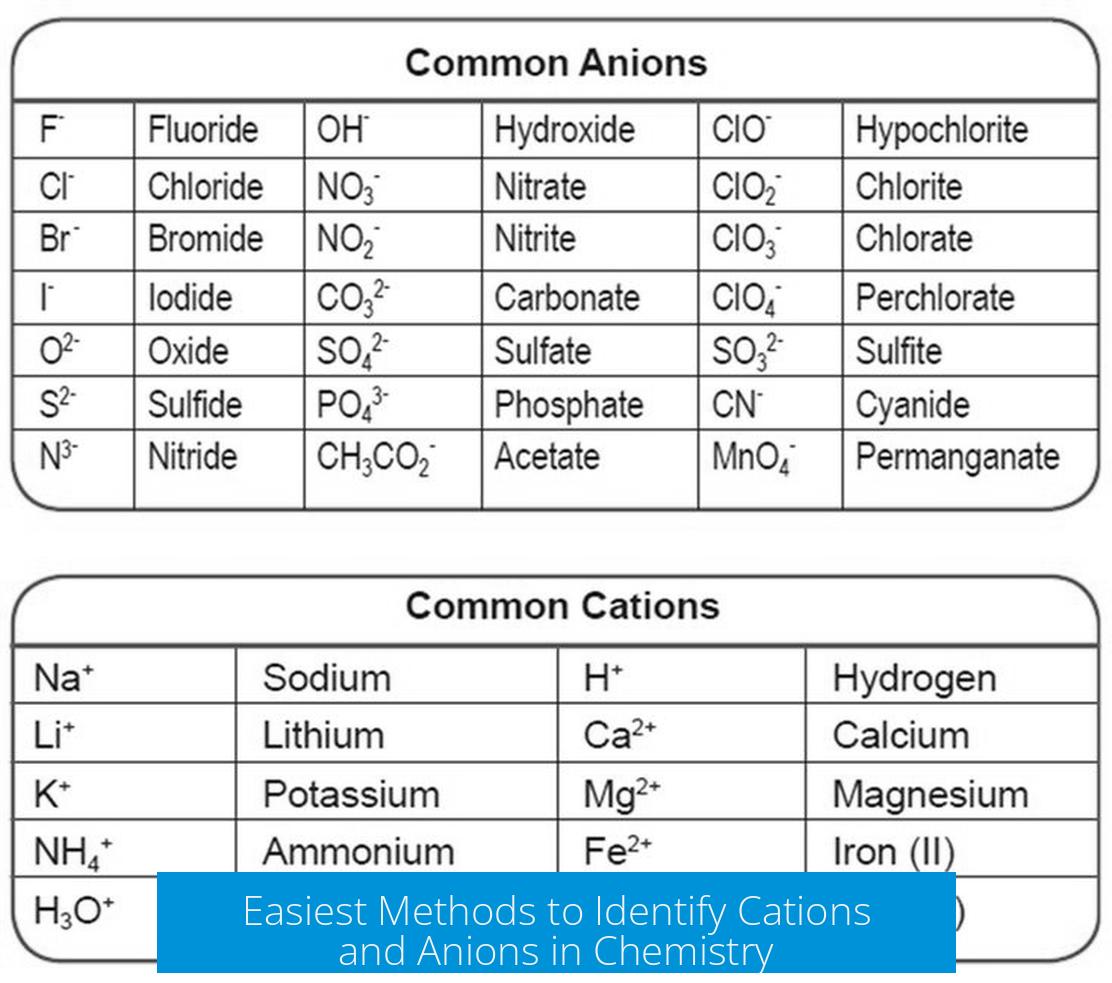
Identifying the charge helps confirm whether an element is acting as a cation or an anion in compounds. For example, sodium (a metal) forms Na+ cations, and chlorine (a nonmetal) forms Cl− anions.
Exceptions and Special Considerations
While the general rule holds, some elements do not fit neatly:
- Elements like carbon and silicon do not commonly form ions independently because their ionization energies and electron affinities make such formations less favorable.
- Theoretically, any element can form a cation or anion depending on the chemical environment and energetic factors.
- Transition metals may form multiple cations with different charges.
Therefore, while classification by metal or nonmetal offers the simplest guide, considering ionization energy and electron affinity can clarify ambiguous cases.
Summary of Key Points
- Metals usually form positively charged cations.
- Nonmetals generally form negatively charged anions.
- Cations have fewer electrons than protons; anions have more.
- Elements like carbon and silicon rarely form ions on their own.
- Ion formation depends on ionization energy and chemical context.
Easiest Way to Identify Which Elements Are Cations and Anions
Ever stared at a periodic table and wondered, “Which ones are cations and which ones are anions?”
In simple terms, cations have a positive charge, and anions carry a negative charge. But how do you know which element becomes which? If you’re tired of confusing ions and need a quick, reliable method, you’ve landed in the right place.
The easiest way to identify which elements are cations and anions is this:
Metals typically form cations, and nonmetals typically form anions. Yep, it’s that straightforward most of the time.
Why Does This Rule Work?
Every element wants to be energetically comfortable, which involves gaining or losing electrons to achieve a stable electronic configuration. Metals are usually on the left and middle of the periodic table. They have few electrons in their outer shell and lose them easily to become positively charged cations.
Nonmetals, found on the right side of the periodic table, have more electrons in their outer shell. They tend to gain electrons to fill their valence shell, becoming negatively charged anions. This simple metal vs. nonmetal distinction guides us effectively through most cases.
Understanding the Charges: Cations vs. Anions
- Cations: Positively charged ions created by losing electrons.
- Anions: Negatively charged ions formed by gaining electrons.
Think of electrons as tiny negative tickets. Losing tickets means a positive balance, so metals give away electrons and become cations. Nonmetals pick up extra tickets, leading to a negative charge — hence, anions.
The Little Curveballs: Exceptions and Special Cases
Of course, nature loves its exceptions.
Take carbon and silicon. These chaps don’t like being classed into neat boxes. They don’t readily form ions on their own. Instead, they often build covalent bonds, sharing electrons rather than handing them over. So, trying to pin cation or anion labels on them is like trying to put on socks while wearing shoes.
Also, technically any element can be either a cation or an anion, if conditions are just right. Ionization energy—the energy required to remove an electron—and electron affinity—the energy change when gaining an electron—play a massive role here.
If it’s energetically favorable for an element to lose an electron, it will form a cation. If gaining an electron is easier, it’ll become an anion. This means some metals in exotic environments can act like nonmetals, and some nonmetals can even lose electrons, though that’s less common.
Hands-On Tips: How to Quickly Spot Cations and Anions
- Look at the periodic table position. If it’s a metal on the left or middle, bet on cations.
- Spot the nonmetals on the right and top corner? They probably form anions.
- Metalloids like boron, arsenic, or antimony might surprise you, so double-check their chemistry before you guess.
- Remember, hydrogen is a wildcard. It mostly forms cations (H+), but under rare circumstances, it can form anions (H−), called hydrides.
For example, sodium (Na) hangs out in group 1 of metals. It’s famous for shedding one electron to become Na+, a neat cation. Chlorine (Cl), a member of the nonmetal halogens, loves to snag an electron, becoming Cl−, a classic anion.
Why Does This Matter Practically?
Understanding whether elements form cations or anions isn’t just academic—it solves real problems.
- Predicting compound formulas. For instance, Na+ pairs with Cl− to form NaCl.
- Deciphering electrical conductivity, as ions are charge carriers.
- Crafting new materials, medicines, and industrial catalysts that depend on ionic behavior.
If you ever find yourself asking “Why does this element behave like a cation or anion?” you’re dipping your toes in some pretty interesting chemistry. Ion charges decide the soup of chemical reactions bubbling around us.
Final Thoughts
So, to recap: The easiest way to identify cations and anions is to check whether the element is a metal or a nonmetal. Metals tend to lose electrons and form positive cations, and nonmetals tend to gain electrons to form negative anions.
Granted, some elements don’t fit neatly into this mold. Carbon and silicon, for instance, don’t typically form ions and prefer sharing electrons instead. Ionization energy and electron affinity explain why some elements might deviate from the norm, reminding us that chemistry always has surprises.
Next time you’re faced with a chemical puzzle, pause and ask: “Is this metal or nonmetal? What charge does it likely carry?” Using this, you unlock a quick mental shortcut to ion identity.
So keep that periodic table handy. It’s not just a chart — it’s your best friend in the quest to quickly spot cations and anions. Chemistry made easy. Who knew?
What is the simplest way to tell if an element is a cation or an anion?
Metals usually form cations, which have a positive charge. Nonmetals tend to form anions, carrying a negative charge.
Why do metals form cations and nonmetals form anions?
Metals lose electrons easily, becoming positively charged. Nonmetals gain electrons, resulting in a negative charge.
Are there elements that do not fit the typical cation or anion pattern?
Yes. Elements like carbon and silicon rarely form ions. Their ion formation depends on energy conditions, making the process less common.
Can any element become either a cation or an anion?
Theoretically yes. Ionization energy dictates if an element prefers to lose or gain electrons. This affects whether it forms a cation or an anion.



Leave a Comment