Rotovap vs Standard Vacuum Distillation
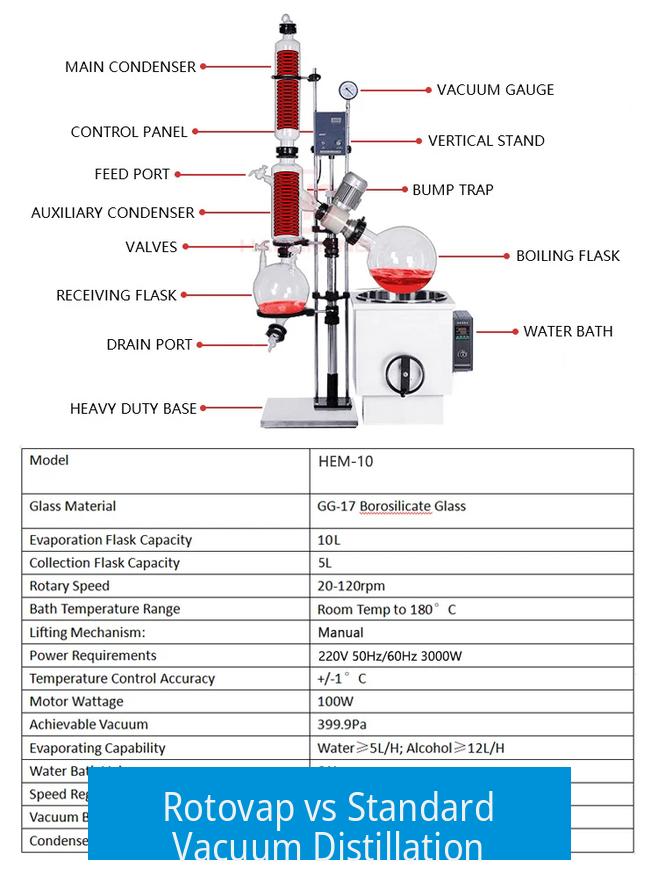
A rotary evaporator (rotovap) is designed primarily to remove volatiles gently and efficiently, rather than to collect or fractionate distillates as a standard vacuum distillation setup does. This fundamental difference shapes their respective applications and limitations.
Purpose and Functionality
- Rotovap: Used mainly to evaporate solvents rapidly under reduced pressure with gentle heating. Ideal for concentrating solutions or removing low-boiling solvents without degrading heat-sensitive compounds.
- Standard Vacuum Distillation: Designed to separate components based on boiling points, allowing precise fraction collection and temperature monitoring.
Key Limitations of Rotovap
- Cannot monitor the distillate temperature for fraction selection during operation.
- Changing collected fractions requires breaking vacuum, disrupting the process.
- Poor efficiency in recovering small distillate volumes below 20–50 mL.
- Does not achieve high vacuum levels needed for volatile or low vapor pressure substances.
- Incapable of maintaining apparatus parts at elevated temperatures to prevent clogging from low-melting solids.
- Cannot operate under inert atmospheres, limiting use for oxygen-sensitive compounds.
Advantages of Rotary Evaporation
- Enables very gentle heating, typically using a water bath capped at about 100°C, reducing risk of overheating.
- Quick and easy attachment or removal of flasks minimizes breakage risk.
- Efficient solvent removal from large volumes, providing almost complete evaporation in many cases.
Additional Benefits
- Faster evaporation rates compared to classical distillation.
- Preserves heat-sensitive compounds due to controlled, gentle heating.
- Improved concentration capabilities for diverse sample types.
- Minimizes chemical degradation during solvent removal.
- Flexibility for handling a range of solvent and sample types.
Disadvantages of Rotary Evaporation
- Higher initial equipment cost compared to standard distillation setups.
- Susceptible to bumping and boilovers, requiring careful control.
Summary Table: Rotovap vs Standard Vacuum Distillation
| Feature | Rotary Evaporator | Standard Vacuum Distillation |
|---|---|---|
| Main Purpose | Remove solvents gently | Separate and collect fractions |
| Temperature Monitoring | No real-time monitoring of distillate | Precise temperature control and monitoring |
| Vacuum Level | Moderate vacuum | High vacuum achievable |
| Fraction Collection | Not practical | Possible without breaking vacuum |
| Handling Small Volumes | Poor recovery below 20–50 mL | Suitable for small volumes |
| Operation under Inert Atmosphere | No | Yes |
| Cost | More expensive | Generally cheaper |
Key Takeaways
- Rotovaps excel at rapid, gentle solvent removal but do not allow fraction collection or fine control of distillate fractions.
- Standard vacuum distillation suits precise separation and collection of fractions with better temperature and vacuum control.
- Rotovap limitations include inability to handle small volumes well, lack of inert atmosphere use, and moderate vacuum range.
- Rotovaps provide gentler heating, faster evaporation, and minimize degradation of sensitive compounds.
- The choice depends on whether solvent removal or precise fractionation is the experimental goal.
What are the main limitations of a rotary evaporator compared to standard vacuum distillation?
A rotary evaporator can’t monitor distillate temperature or collect different fractions without breaking vacuum. It struggles with small volumes, low volatility compounds, and can’t operate under inert atmosphere or maintain apparatus warmth for low-melting solids.
Why is rotary evaporation considered gentler for heat-sensitive compounds?
Rotovaps use gentle heating, usually with a water bath maxing near 100°C. This low heat reduces degradation and protects sensitive samples during solvent removal or evaporation steps.
Can a rotary evaporator handle fraction separation like standard vacuum distillation?
No, rotovaps aren’t designed for fraction collection. Their setup removes volatiles but doesn’t allow fraction switching without breaking the vacuum, unlike classical vacuum distillation which can separate fractions based on boiling points.
What are advantages of rotary evaporation in workflow and solvent removal?
Rotovaps allow quick flask attachment and detachment, minimizing breakage risk. They evaporate solvents faster and more efficiently, especially for heat-sensitive samples, leading to better concentration and reduced compound degradation.
Are rotary evaporators cost-effective compared to classical vacuum distillation?
They tend to be expensive and can suffer from boil overs or bumping. Despite this, their ease of use and efficient evaporation often justify the cost in many lab settings.





Leave a Comment