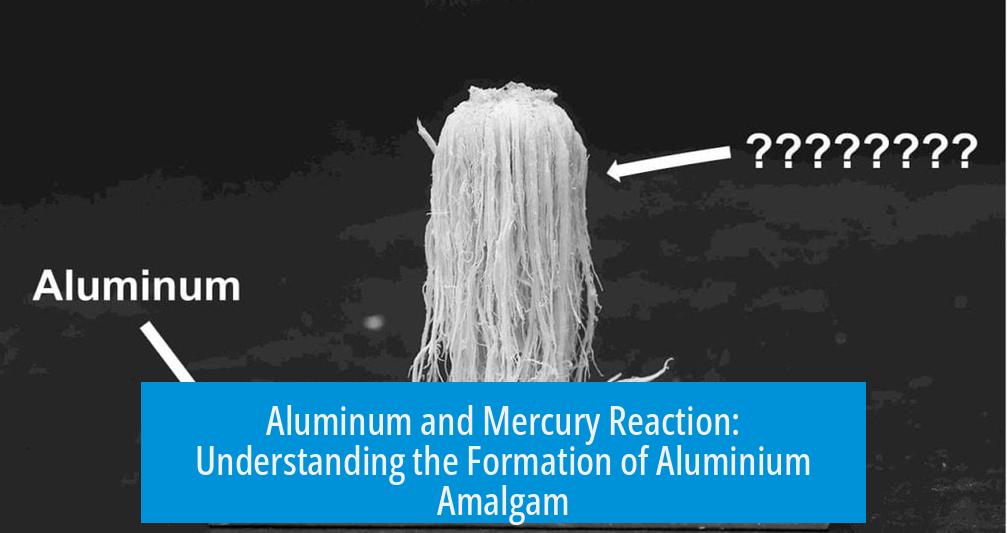
Aluminum and Mercury Reaction: Formation of Aluminium Amalgam

When aluminum comes into contact with mercury, it forms an alloy known as aluminum amalgam. This reaction creates a visually captivating process that continues as long as mercury contacts aluminum. The interaction involves mercury breaking down aluminum’s protective oxide layer, enabling the metals to merge.
Chemical Reaction and Mechanism

Aluminum naturally forms a thin, tough layer of aluminum oxide (Al2O3) on its surface, protecting it from corrosion. Mercury disrupts this oxide layer. When mercury contacts aluminum, it penetrates and amalgamates with metallic aluminum beneath the oxide. This results in aluminum amalgam, a soft yet reactive alloy.
The aluminum oxide appears as white crystals during this process. Mercury’s role is vital: it allows aluminum to react more actively by exposing fresh metal surfaces. This makes the reaction unique and ongoing as the surface keeps renewing.
Observations and Public Interest
- This reaction has gained popularity on educational platforms such as YouTube. Video creators, including NileRed, demonstrate its fascinating nature with clear visual effects.
- Visual appeal lies in the persistent, spreading action of mercury over aluminum surfaces.
- People often find it exciting because amalgamation is rarely seen in general chemistry labs.
Safety Considerations and Environmental Concerns

While the reaction is intriguing, handling mercury requires extreme care due to its toxicity. Mercury vapors pose significant health risks, leading to strict regulations on its transport and use (for example, mercury is banned on aircraft).
Disposal of mercury-containing waste, including aluminum amalgam residues, demands specialized procedures to avoid environmental contamination. Such reactions should only occur in controlled laboratory settings, never casually performed outside professional environments.
Common Misconceptions
There is confusion between mercury amalgams and gallium alloys used in dentistry or other applications. Mercury was indeed used historically in dental fillings but not interchangeably with gallium, which can also interact with aluminum in separate contexts.
Industrial Impact
Unexpected contact with mercury can damage aluminum components in industries. For example, brazed aluminum heat exchangers suffer from thermal cycling failures and leaks when exposed inadvertently to mercury or similar amalgamating agents.
Key Takeaways
- Aluminum amalgam forms when mercury disrupts aluminum oxide, allowing alloying.
- Aluminum oxide manifests as white crystals during the reaction.
- The reaction is visually striking and educative but hazardous due to mercury toxicity.
- Proper safety measures and disposal protocols are crucial when handling mercury.
- Amalgamation is distinct from other metal interactions like those involving gallium.
- Industrial equipment using aluminum can be compromised by mercury exposure.
Aluminum + Mercury = Aluminium Amalgam: The Wild Chemistry You Didn’t Know You Needed
Aluminum + Mercury = Aluminium Amalgam. Sounds simple, right? But this quirky chemical mix has a story that goes way beyond the periodic table. It’s a reaction that’s caught many eyes online and left even chem enthusiasts scratching their heads in awe. Let’s unpack this fascinating reaction from the spark of visual pop to its lurking hazards, and industrial headaches.
Picture this: a video goes viral — aluminum, the shiny silvery metal you see in every soda can, meets mercury, that dense, silvery liquid metal. When they come into contact, mercury breaks down aluminum’s thin oxide layer, forming an ‘amalgam.’ This shiny mash-up isn’t just for show; it invites a continuous, lively reaction that keeps fizzing and cracking. Just like that, a chemical spectacle unfolds, and the internet can’t get enough.
The Sizzle Behind the Spectacle
Have you ever stumbled upon a reaction that just refuses to quit? This one fits the bill perfectly. The aluminum dissolves into the mercury, creating a sticky, silver goo that seems to dance constantly. Videos by NileRed, a YouTube chemist phenom, showcase this in glowing detail — no surprise his 2017 video remains a gold mine for curious minds. The white crystals you see bordering the mixture? That’s aluminum oxide, the stubborn skin that mercury breaks through to get to the aluminum beneath.
Watching this reaction is like watching slow-motion fireworks for metal lovers. It captivates because it defies the usual stoic image of metals. The amalgamation process demonstrates nature’s beautiful complexity in a smooth, continuous dance. Chemistry is, indeed, wonderful.
Why Do People Care? Because Science Meets Art
This reaction isn’t just entertainment. It’s a practical demonstration of surface chemistry and metal behavior. Amalgams have historical and modern significance. For example, mercury amalgams have been used in dental fillings for decades—though that’s mostly mercury with silver, not aluminum. Such details stir up confusion; many mix up mercury amalgams with gallium’s ability to melt aluminum. So, if someone asks if “this goes in your teeth,” clarify: “That’s mercury and silver, not aluminum.”
Warning: Not Your Kitchen Experiment
Before you rush to grab some mercury, a cautionary note. Mercury is nasty stuff, highly toxic, and strictly controlled. You can’t even bring it onto an airplane. The fumes and waste from these reactions pose serious disposal problems. Ever wonder what to do with leftover mercury or the amalgam sludge? It doesn’t just vanish safely down the drain. Laboratories follow strict protocols—strictly avoid trying this at home.
So, admire the videos, appreciate the chemistry, but leave the mercury to the professionals. Besides, the hazards don’t stop there. Industrial encounters with aluminum amalgam can be downright scary.
Industrial Drama: When Chemistry Plays Hardball
It’s not all sci-fi experiments. Aluminum amalgamation has real impacts in industry, especially in brazed aluminum heat exchangers (BAHX). These devices keep power plants, HVAC systems, and automotive cooling running smoothly—until mercury contamination sneaks in. Mercury attacks the aluminum surfaces, causing leaks and failures that can escalate dramatically. One worker’s experience: “Had a major leak, and it was a nightmare trying to fix. These exchangers are my least favorite gear now.” Think of it as a tiny saboteur slowly eroding metal strength.
Thermal cycling — heating and cooling repeatedly — only worsens the damage, bending metal like a soft soda can. No surprise that companies push guidelines to avoid mercury contamination at all costs. Safety in industry isn’t just about preventing explosions; it’s about preserving equipment against sneaky chemical enemies.
Fun Fact or Experimental Curiosity?
Some science experimenters wonder if changing gases around the reaction changes the aluminum amalgam’s behavior. Could this be a new frontier in materials science? Unfortunately, mercury’s toxicity leaves most to just marvel at videos, like NileRed’s, without splashing into the lab themselves. The phrase “that’s metal erection” (yes, that’s a joke chemists like) captures the quirky spirit around this metal-on-metal action.
Final Thoughts: A Reactivity Full of Contrasts
Aluminum + mercury doesn’t create just a metallic blob. It creates a mesmerizing chemistry show with serious lessons. It mixes beauty and risk, science and safety. It sparks creativity, cautious handling, and curiosity about metals’ hidden secrets. If you ask what makes this reaction so cool, the answer isn’t just the fizz or crackle; it’s how such a simple pairing unlocks an elaborate chain of physical, chemical, and even industrial stories.
So next time you come across a shiny aluminum can or hear about mercury’s role in history, remember their alliance in the aluminum amalgam — a small but mighty testament to chemistry’s ongoing wonders and warnings.
Looking to dive deeper? Check out NileRed’s definitive video here: https://youtu.be/IrdYueB9pY4. It’s where science meets spectacle. Just, you know, keep the mercury out of your luggage.




Leave a Comment