Are Ions a Type of Atom?
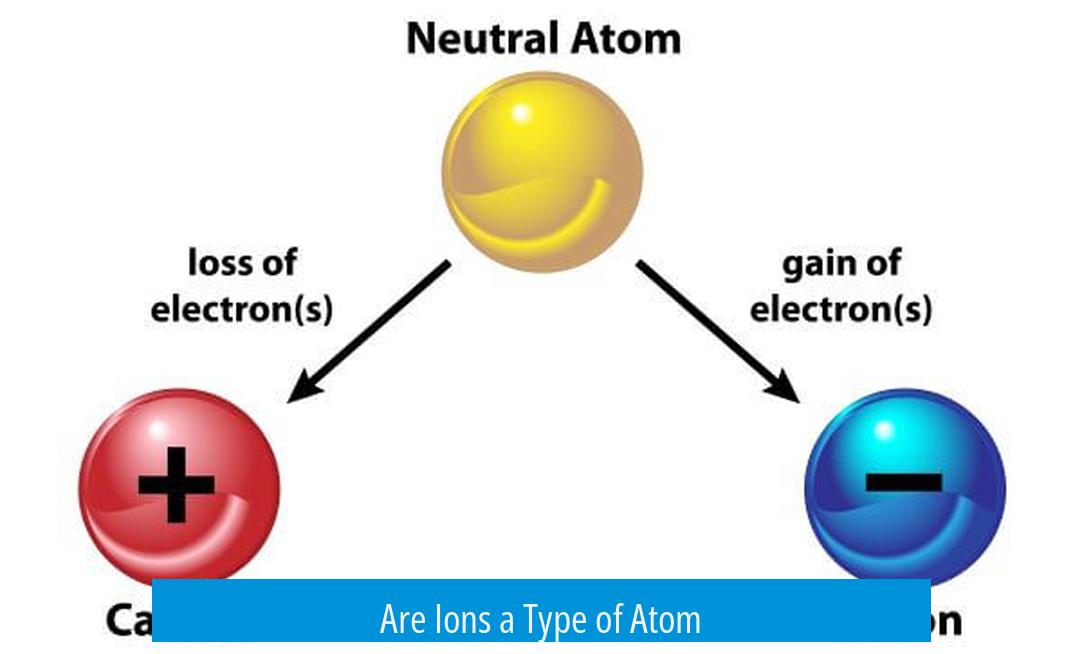
Ions are atoms or molecules that carry a net electric charge due to the gain or loss of electrons, making ions variations of atoms rather than entirely different entities. This fundamental concept helps clarify the relationship between atoms and ions, essential in understanding chemical behavior.
Understanding the Relationship Between Ions and Atoms
Atoms usually have an equal number of protons (positively charged) and electrons (negatively charged), resulting in a neutral overall charge. When this balance changes, the atom becomes an ion. For instance, sodium chloride (NaCl) dissolves in water and splits into Na+ and Cl− ions, which are atoms missing or gaining electrons. Hence, ions are best described as atoms with a charge imbalance.
- Atoms maintain the same number of protons defining their element identity (e.g., iron always has 26 protons).
- Ions arise when atoms gain or lose electrons but keep their proton count. For example, an iron atom missing one electron forms a positively charged ion (Fe+).
- Monoatomic ions are single atoms carrying either a positive or negative charge.
Thus, ions are not fundamentally different from atoms but represent electronically charged variants. The charge changes chemical properties yet retains the core atomic structure.
Monoatomic vs. Polyatomic Ions
While many ions consist of a single charged atom (monoatomic ions), some are composed of multiple atoms bonded together. These are called polyatomic ions.
| Type of Ion | Composition | Example |
|---|---|---|
| Monoatomic Ion | One atom with charge | Na+, Cl−, Fe3+ |
| Polyatomic Ion | Multiple atoms bonded together carrying net charge | CO32− (carbonate), SO42− (sulfate), NH4+ (ammonium) |
Polyatomic ions consist of atoms from different elements bonded covalently but collectively bearing charge by gaining or losing electrons. Therefore, not all ions are atoms. Molecules can become ions when their total electron count shifts.
Charge Status: Atoms vs. Ions
Atoms maintain neutral electrical charge under normal circumstances. Ions form when atoms or molecules:
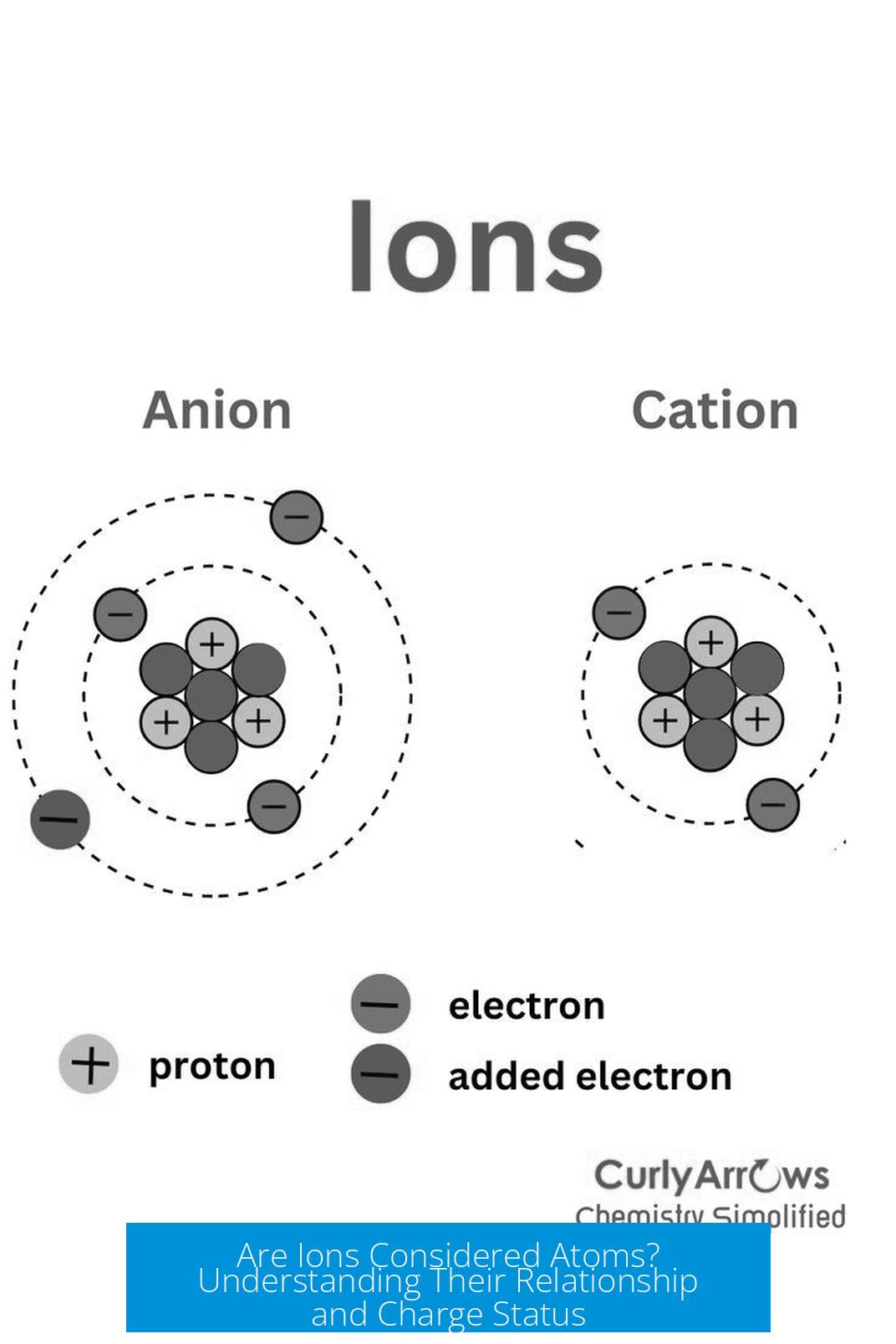
- Gain electrons -> become negatively charged ions called anions.
- Lose electrons -> become positively charged ions called cations.
Examples include:
- Cl−: Chlorine atom gains an electron, becomes an anion.
- Na+: Sodium atom loses an electron, becomes a cation.
This electrical charge influences chemical reactivity and interactions like ionic bonding.
Conceptual Analogies: Why Ions Are Still Atoms
Imagine an atom as an individual who normally has a balanced set of possessions (electrons). Losing or gaining electrons is like putting on or removing clothing items—you remain the same person but with a temporary change.
In chemical terms:
- Atoms are the base unit, neutral by default.
- Ions are these atoms with added or missing electrons.
- This does not change the elemental identity, just the charge state.
Hence, ions and atoms share the same atomic core. Ionization is a reversible process, similar to altering outer conditions while keeping the fundamental identity intact.
Atoms as the Minimum Units That Can Be Ionized
Atoms serve as minimum units capable of ionization. Molecular compounds, made from atoms, can also ionize as groups (forming polyatomic ions), but individual atoms themselves may be ionized directly.
- Atoms are building blocks forming molecules and ions.
- Ions are either individual atoms or clusters (polyatomic ions) with net charge.
This fundamental understanding is central to chemistry, impacting fields like electrochemistry, biochemistry, and materials science.
Summary of Key Points
- Ions are atoms or molecules carrying net electric charge due to gain or loss of electrons.
- Monoatomic ions are charged atoms; polyatomic ions contain multiple atoms.
- Atoms retain their identity through fixed proton numbers even when charged.
- Positive ions (cations) result from electron loss; negative ions (anions) result from electron gain.
- Ions differ in charge but remain fundamentally the same atoms or molecules.
Are ions considered a type of atom?
Yes, monoatomic ions are atoms with a positive or negative charge. They differ from neutral atoms by having gained or lost electrons, but they remain the same element.
How do ions differ from neutral atoms?
Atoms have an equal number of protons and electrons, making them neutral. Ions have lost or gained electrons, giving them a net positive or negative charge.
Can ions consist of more than one atom?
Yes, some ions are polyatomic, meaning they contain multiple atoms bonded together with an overall charge, like CO3 2-, which is not a single atom but a charged molecule.
Is the identity of an ion the same as that of its neutral atom?
Yes. An ion retains the same number of protons as its neutral atom, so it remains chemically the same element, just with a charge caused by electron gain or loss.
Are atoms the smallest units that can become ions?
Atoms are the smallest units that can be ionized. Molecules can also carry charge and become polyatomic ions, but atoms represent the minimum unit when talking about ionization.



Leave a Comment