Understanding Compounds vs Molecules
A compound is a substance made of two or more different elements chemically bonded, while a molecule is a group of atoms bonded covalently, regardless of whether they are the same or different elements.
Definitions and Key Differences
Compound

- Defined as a substance formed by multiple different elements chemically bonded.
- Can be ionic or covalent in nature.
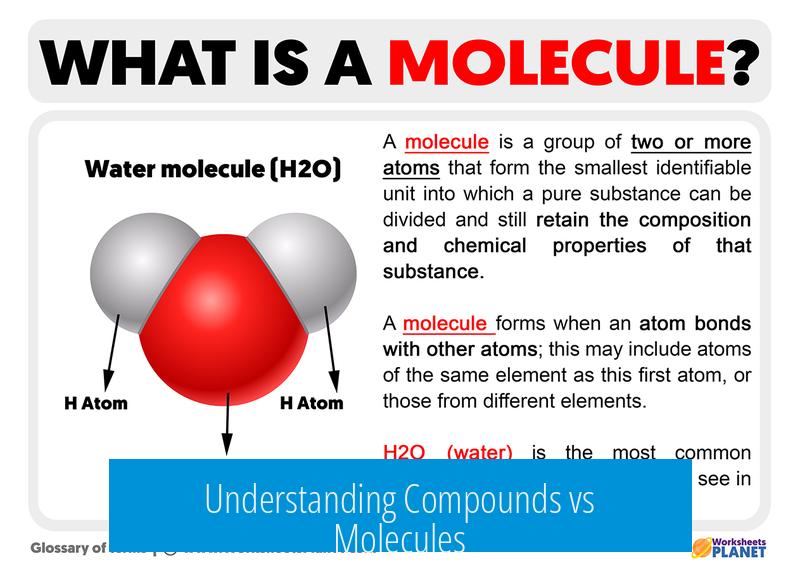
- Example: Water (H2O) is a compound composed of hydrogen and oxygen.
Molecule
- Refers to atoms chemically bonded by covalent bonds.
- Can be elemental or compound molecules.
- Not all molecules are compounds; e.g., O2 and N2 are molecules but not compounds.
Relationship Between Compounds and Molecules
The key relationship is that compounds consist of molecules made from different elements combined. Molecules can be elemental, like O2, or made from multiple elements, like in water.
The term “molecule of a compound” helps reduce confusion when discussing substances. While people often use “compound” and “molecule” interchangeably, the distinction matters for clarity.
Special Cases and Examples
Diatomic Molecules Not Compounds
- Elements such as nitrogen (N2), hydrogen (H2), oxygen (O2), and fluorine (F2) occur naturally as diatomic molecules.
- These are molecules but not compounds because they contain only one element.
Allotropes of Carbon
- Forms such as diamond, graphite, and buckminsterfullerene consist of only carbon atoms linked covalently.
- These are molecular networks but not compounds since they do not have different elements combined.
Summary of Key Points
- A compound contains two or more different elements chemically bonded.
- A molecule is a group of covalently bonded atoms, either elemental or compound.
- Diatomic molecules of a single element are molecules but not compounds.
- Common molecules like water are molecules and compounds simultaneously.
- Clear distinction avoids ambiguity between molecular and compound substances.
Compounds vs Molecules: What’s the Real Difference?
Are compounds and molecules the same thing? The short answer: No, but they’re closely related. The confusion here is so common it might make your head spin faster than a chemistry exam question. Let’s unravel this with clear, digestible details.
Imagine you’re at a party filled with tiny atoms. Some atoms just hang out with their own kind — like oxygen atoms bonding to oxygen atoms to form O2. Others mix and mingle with different atoms, such as hydrogen bonding with oxygen to make H2O, aka water. Here lies the essence of the difference.
1. Definitions and Distinctions: What’s a Compound? What’s a Molecule?
First, a compound is a substance made of two or more different elements chemically bonded. Picture it as a recipe — you need different ingredients (elements) to create that complex dish called a compound. The magic comes in two flavors: covalent compounds (where atoms share electrons) and ionic compounds (where atoms transfer electrons).
Water (H2O) is the poster child of compounds. It’s made of two hydrogen atoms and one oxygen atom combined covalently. This means it’s a compound, formed by distinct elements.
A molecule, on the other hand, is any group of atoms bonded together covalently. These atoms may be the same element or different ones. This distinction is crucial. If atoms in a molecule are all the same, like in oxygen gas (O2), that’s just a molecule but NOT a compound. So, molecules include compounds, but not all molecules qualify as compounds.
Put simply: all compounds are molecules, but not all molecules are compounds. To avoid confusion, chemists might say “molecule of a compound” to describe compounds more accurately.
2. Relationship Between Compounds and Molecules — The Chemistry BFFs
The relationship? Think of molecules as clumps of atoms bonded covalently. When those atoms are different elements, you form a compound. Water is an excellent example: a compound made up of molecules.
However, calling a molecule a compound can sometimes lead to ambiguity. If you say “molecule” alone, you might capture both single-element molecules and multi-element compounds. Without context, it’s like saying “fruit” — is it an apple or a banana? Both are fruit, but they have clear differences.
Confession time: even seasoned chemists don’t always stress over this fuzziness. The distinction, after all, is context-dependent. In many scenarios, the loose interchange of “molecule” and “compound” is fine, as long as you understand what you mean.
3. Special Cases and Examples: When the Lines Get Blurred
3.1 Diatomic Molecules: Molecules That Aren’t Compounds
Some elements break the mold by existing naturally as diatomic molecules—two atoms bonded together, but both atoms are the same element. Think about nitrogen (N2), oxygen (O2), hydrogen (H2), and fluorine (F2). These are molecules by definition but certainly not compounds because there’s no combination of different elements.
This phenomenon highlights how molecules and compounds are not identical concepts. It helps clarify that molecules are more broadly defined.
3.2 Allotropes of Carbon: Not Compounds, Even with Complex Bonds
Carbon throws another twist with its allotropes — different structural forms of the same element. Diamond, graphite, and buckminsterfullerene (scientists’ fancier name for buckyballs) have networks of covalent bonds connecting carbon atoms.
Despite the complexity and extensive bonding, these allotropes are not compounds since they consist solely of carbon atoms. They are molecules of a single element, showcasing how molecular structures can be sophisticated without forming compounds.
So, Why Does It Matter?
Understanding the difference can help you decode chemistry texts and fall less prey to misleading jargon. When you read “compound,” expect multiple elements bonded together. “Molecule” means bonded atoms — same or different.
This knowledge proves useful from high school labs to real-world applications like drug design, where precise molecular combinations create life-saving drugs (compounds). Or in environmental science, where oxygen molecules (O2) sustain life but aren’t compounds.
Here’s a practical tip: when discussing chemistry, be clear whether you talk about molecules in general or specifically about compounds. It makes your communication sharper and more scientifically sound.
Wrapping Up: The Chemistry Tango Between Compounds and Molecules
Molecules and compounds are dance partners, but with different moves. Molecules are groups of atoms stuck together by covalent bonds; compounds are molecules made of different elements. Some molecules refuse to be called compounds — like O2 or C diamonds.
Next time you stumble on this chemistry puzzle, remember:
- A molecule is a team of atoms bonded together, no matter if they’re all the same kind.
- A compound is a molecule involving different types of atoms in harmony.
- Diatomic molecules and allotropes prove nature loves exceptions.
Are you now feeling more confident to spot a molecule or compound in the wild? Chemistry can be tricky, but a solid grasp of these terms makes a huge difference. So, get out there and identify those molecules and compounds like a science pro!
What is the main difference between a compound and a molecule?
A molecule is a group of atoms joined by covalent bonds. A compound is a substance made from different elements combined chemically. All compounds are molecules, but not all molecules are compounds.
Can a molecule exist without being a compound?
Yes. Molecules of a single element, like O2 or N2, consist of atoms bonded covalently but are not compounds since they contain only one type of element.
Are all compounds made of molecules?
No. Compounds can be ionic or covalent. Ionic compounds do not form molecules but exist as a lattice of ions. Covalent compounds form molecules by sharing electrons.
Why are allotropes like diamond not considered compounds?
Allotropes of carbon are pure elements with atoms bonded covalently in networks. Since they contain only one element, they are molecules or structures but not compounds.
Is water a molecule or a compound?
Water is both. It is a molecule because it has atoms bonded covalently. It is a compound because it consists of two different elements, hydrogen and oxygen.



Leave a Comment