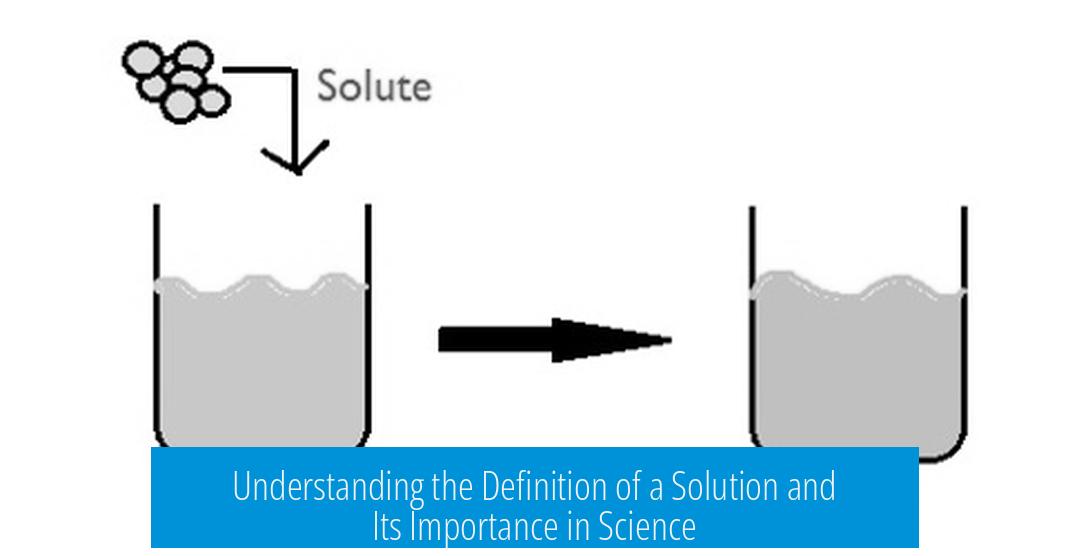
Definition of a Solution

A solution is a homogeneous mixture of two or more substances where one substance (solute) is uniformly dispersed within another (solvent) in a single phase. This definition applies mainly to liquid and solid phases. Solutions have consistent composition and properties throughout.
Existence of Solutions in Different Phases

Solutions in the Gas Phase

There are no true solutions in the gas phase. What are often mistaken for gas solutions are gas mixtures. Gas molecules move independently with minimal interaction. This behavior means gases do not form homogeneous solutions like liquids and solids do.
Misconceptions About Gas Mixtures

A frequent misunderstanding involves “the solution of water in air.” This phrase is incorrect. Air is a mixture of gases and water vapor coexists but does not dissolve in air as in a liquid solution.
Behavior of Gas Mixtures vs. Solutions

Gas Mixtures
The gaseous phase is better described as a gas mixture. Each gas component maintains its own physical properties. Partial pressures of gases combine without changing individual molecular identities.
Relationship Between Partial Pressure and Vapor Pressure
A relative humidity of 100% means the partial pressure of water vapor equals the water’s vapor pressure. At this point, liquid water and water vapor are in equilibrium, not indicating that air dissolved as much water as possible.
Molecular Interactions in Gas Phase
Molecules in the gas phase do not significantly interact with each other. This lack of interaction prevents gas mixtures from forming true solutions, which require uniform molecular interaction.
Key Takeaways
- Solutions are homogeneous mixtures existing typically in liquid and solid phases.
- There are no solutions in the gas phase; gas mixtures differ fundamentally from solutions.
- “Solution of water in air” is a misconception; water vapor and air coexist without true dissolution.
- Gas molecules exhibit minimal interaction, maintaining individual identities and partial pressures.
- 100% relative humidity means equilibrium between liquid water and vapor, not maximum dissolution in air.
What’s the Definition of a Solution and Why Gas Phase Doesn’t Cut It
Hit pause if you ever thought mixing water in air creates a “solution.” Spoiler alert: it doesn’t. Let’s dive into what a solution really is and why gas-phase “solutions” are, well… not really solutions.
The Straight Answer: What Exactly Is a Solution?
A solution is a homogeneous mixture where a solute is completely dissolved into a solvent, generally involving molecular or ionic interactions in liquid or solid phases. Simply put, the molecules of solute and solvent mix uniformly and interact at a microscopic level.
But hold on… before you say, “That means air with water vapor must be a solution!” — let’s unpack that.
Gas Phase? No Solutions Here.
Despite what the casual language suggests, gas mixtures aren’t solutions. A common mistake happens when people say, “there’s a solution of water in air.”
Fact check: there are no solutions in the gas phase. You’re actually looking at a gas mixture, not a solution.
Why? Because the molecules in a gas phase are pretty much doing their own thing. They don’t interact enough to qualify as a solution. Molecular forces are weak, and gases mix simply by volume, not via dissolving.
Gas Mixtures: More Like Roommates, Less Like Friends
Think of molecules in a gas mixture as roommates sharing an apartment but without communicating or bonding deeply.
- They don’t form bonds or interact strongly.
- Each gas keeps its identity—oxygen stays oxygen, nitrogen stays nitrogen, and water vapor stays water vapor.
This contrasts with a true solution, like sugar dissolved in water, where sugar molecules disperse evenly and interact with water molecules.
Partial Pressure and Vapor Pressure: The Real Story Behind “Saturation”
Ever wondered what 100% relative humidity means? Does it mean air can’t hold any more water? Not quite.
When humidity hits 100%, the partial pressure of water vapor equals its vapor pressure at that temperature. This sets an equilibrium between liquid water and water vapor—neither evaporates nor condenses faster than the other.
So, it’s not that air “saturates” and becomes a solution of water and air. Instead, liquid water and water vapor co-exist in balance.
The Importance of Molecular Interactions
What makes solutions special? It’s molecular interaction—bonds or attractions between solute and solvent molecules.
In liquids or solids, molecules feel each other. They attract, repel, and weave into a unified mixture.
Gases don’t bother. They mostly ignore each other, floating, bumping occasionally, but never forming lasting bonds. This mismatch is why gas mixtures lack solution status.
Why Definitions Matter in Science and Everyday Life
So why get strict about calling a gas mixture a “solution?” Precision counts.
In chemistry and physics, mixing gases is a physical combination, not a chemical or molecular interaction-based solution. Misusing terms can confuse scientific communication or cause misunderstanding in practical scenarios like meteorology or engineering.
For example, engineers designing air conditioning systems must know that humidity involves gas mixtures, not solutions, affecting condensation and evaporation calculations.
How Can This Help You? Practical Tips and Example
- Next time you hear “water dissolved in air,” pause. Realize this is a gas mixture with water vapor, not a solution.
- If you’re studying solutions, focus on liquids and solids. Notice how sugar, salt, or alcohol dissolve interactively, unlike gases.
- Understand humidity as a balance of pressures. This insight helps grasp weather reports and forecasts better.
A Bit of Real-World Context
Imagine you’re hiking early morning. The fog? Not a solution of water and air. It’s tiny liquid droplets suspended in the air—an aerosol, a suspension, not a solution.
Or consider soda: CO2 gas dissolves in water under pressure, making a proper solution—or more accurately, a physical solution—because CO2 interacts with water molecules. But the free CO2 gas above the soda is just gas mixture, not a solution.
Wrapping It Up: Clarity Creates Confidence
To nail the definition of a solution, keep this in mind:
- Solutions require molecular interaction and uniform mixing.
- They mainly occur in liquid or solid phases.
- Gas mixtures do not qualify as solutions—molecules just coexist, not interact deeply.
- Humidity and related terms describe equilibrium of partial and vapor pressures, not solutions.
Now, when someone casually claims “water dissolves in air,” you can smile knowingly and explain why it’s not a solution but a gas mixture, equilibrium, and molecular independence.
Understanding this subtle but crucial difference sharpens your science savvy and helps decode everyday phenomena with confidence.




Leave a Comment